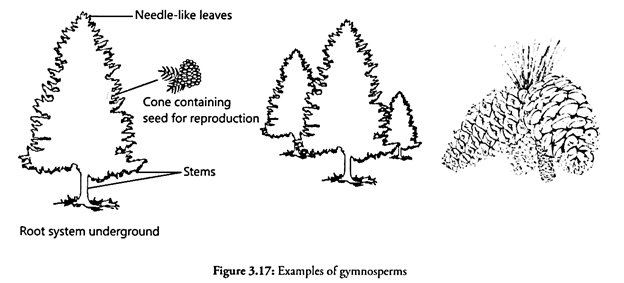
Let us make an in-depth study of the protein targeting. After reading this article you will learn about: 1. Introduction to Protein Targeting 2. Signal Sequence 3. Transport of Proteins into ER 4. Signal Sequence Recognition Mechanism 5. Role of Golgi Complex in Protein Transportation 6. Transport of Proteins from Golgi to Lysosomes 7. Targeting of Proteins to Mitochondria and Chloroplasts 8. Protein Targeting to Chloroplasts 9. Protein Targeting into Nucleus and 10. Membrane Proteins.
Introduction to Protein Targeting:
A typical mammalian cell may contain numerous kinds of proteins and numerous individual protein molecules. The eukaryotic cell is a multi-compartmental structure. Its many organelles each requires different proteins. Except a few of them which are synthesized in mitochondria and chloroplasts all other proteins necessary for the cell and the ones to be secreted by the cell are synthesized in the cytosol on free ribosomes and on ribosomes bound to the endoplasmic reticulum.
Most proteins are coded by the nuclear genome and synthesized in the cytoplasm. The proteins are present in the ER, mitochondria, chloroplasts, Golgi, peroxisomes, nucleus, in the cytosol and in the membranes of all these organelles. They are selectively transported into their appropriate organelles inside the cell and across the plasma membrane to be secreted outside the cell.
Some of them are carried into membrane bound vesicles which bud off from one organelle and transported in definite pathways. Different destinations of different proteins require sophisticated system for labelling and sorting newly synthesized proteins and ensuring that they reach their proper places. This transportation of proteins to their final destinations is called protein targeting.
Proteins destined for cytoplasm and those to be incorporated into mitochondria, chloroplasts and nuclei are synthesized on free ribosomes in the cytoplasm. Proteins destined for cellular membranes, lysosomes and extracellular transport, use a special distribution system. The main structures in this system are the rough endoplasmic reticulum (RER) and Golgi complex.
The RER is a network of interconnected membrane enclosed vesicles or vacuoles. The endoplasmic reticulum is coated with polyribosomes to give it a rough appearance. The golgi complex is also a stack of membrane bound sacs but they are not interconnected. The golgi complex acts as a switching center for proteins to various destinations.
Proteins to be directed to their destinations via Golgi complex are synthesized by ribosomes associated with endoplasmic reticulum.
Signal Sequence:
Protein sorting requires proper address labels which are in the form of peptide signal sequences. A signal sequence that directs the protein to its target is present in the form of 13-35 amino acids in the newly synthesized protein itself. It is the first to be synthesized and is mostly present at the amino N-terminal, sometimes at the carboxyl C- terminal.
It is known as signal sequence or leader sequence. Some proteins are further sorted to a sub-compartment within the target organelle. For this purpose, a second signal sequence is present behind the first signal sequence which is cleaved.
Proteins carried inside the membrane bound vesicles are called cargo proteins. An embedded or integrated protein is carried in the membrane of the vesicle, while secretory protein is carried within the lumen of the vesicle. The vesicle buds off from the donor surface and fuses with the target surface releasing its contents into the target organelle and the membrane protein is incorporated into the membrane of the target organelle. The process is repeated during the passage of protein from ER to Golgi to lysosomes and from Golgi to plasma membrane.
Transport of Proteins into ER:
A short N-terminus signal sequence at the beginning of the growing nascent protein chain’ determines whether a ribosome synthesizing the proteins binds to ER or not. The protein synthesis always begins on free ribosomes. As the signal sequence emerges out of the ribosome, the large ribosomal sub-unit binds to ER membrane.
This is decided by the type of signal sequence. This is the first sorting as the ribosome binds to ER, forming rough ER. Translocation takes place into the ER while growing chain is still bound to the ribosome. This is called co-translational translocation. The process is facilitated by the signal sequence recognition mechanism.
Signal Sequence Recognition Mechanism:
It consists of a signal recognition particle (SRP) present in the cytosol. SRP binds to the signal sequence of the nascent protein as soon as it emerges out of ribosome and directs it towards the ER membrane. The binding of SRP stops further synthesis of protein chain when it is about 70 amino acids long.
This prevents it from folding. The SRP-ribosome complex binds to the SAP receptor, which is an integral membrane protein in the wall of ER and is a docking protein of the ER. At this point GTP hydrolysis hydrolyses frees SRP which is ready for the next round of directing next nascent protein of ER.
Now lengthening of nascent polypeptide restarts which enters ER lumen. Ribosome is aligned to a channel in the wall of ER. This channel is called translocon. It allows the elongating chain to enter the translocon into the ER lumen.
As the growing polypeptide chain emerges into the ER lumen, the signal sequence is cleaved by a peptide called signal peptidase. Inside the lumen, the protein may become folded into its final active form or may be carried into its secretary pathway or may be embedded in the ER membrane.
Once inside the lumen of ER, the protein undergoes folding and several modifications for which the ER lumen contains a number of enzymes and chaprone proteins. The most common processing is glycosylation which involves addition of carbohydrates to the protein chain. Glycosylation generally occurs in the ER lumen but sometimes in Golgi also.
Most oligosachharides or glycons are attached to the amino group NH3 and the proteins are called N-linked glycoprotiens e.g. oligosachharide attached to aspargine. A preformed oligosachharide is added to the proteins. This structure is Man 9 (Glc NAC)2 called high mannose structure.
This contains mannose, glucose and N-acetylglucosamine). All nascent proteins start the sorting pathway by addition of the same pre-formed oligosachharide in plants and animals. Almost all proteins that enter the secretary pathway are glycosylated.
In ER lumen, after glycosylation, many protiens are folded and stabilized by disulphide proteins bonds (-S-S-). This reaction is catalyzed by an enzyme, protein disulphide isomerase (PDI). Most of human proteins are stabilized by disulphide bonds.
Role of Golgi Complex in Protein Transportation:
The role of Golgi complex is to act as a switching center for proteins to various destinations. Both ER and Golgi apparatus are flattened cisternae. Transport of proteins from one compartment (donor) to the next one (target) is carried out in transport vesicles. The vesicles contain cargo proteins in their lumen and integral membrane proteins in their membranes.
The vesicles bud off from ER and fuse with the cis-compartment or receiving compartment of Golgi. In this process cargo proteins are delivered into the lumen of Golgi and membrane proteins become part of the membrane of the target vesicles. The proteins are glycosylated, folded, modified and sorted in ER. This process of glycosylation, modification and sorting of proteins continues in successive Golgi cisternae.
Starting from the cis-compartment to medial compartment and lastly to trans-Golgi network proteins are exported to the end target. In trans-golgi network (TGN) proteins are further sorted to be delivered to lysosomes, for secretion outside the cell and to plasma membrane according to signals present in the nascent proteins.
Transport of Proteins from Golgi to Lysosomes:
The lysosomal enzymes and lysosomal membrane proteins are synthesized in rough ER and transported to Golgi cisternae and ultimately to lysosomes. The sorting signal that directs the lysosomal enzymes from the trans- Golgi network (TGN) to lysosomes is mannose 6-phosphate (M6P). The attachment of M6P to lysosomal enzymes prevents their further modification.
Separation of M6P bearing lysosomal enzymes from other proteins takes place in TGN. The wall of TGN contains M6P receptors. These M6P receptors bind to lysosomal proteins. The vesicles containing these receptor bearing proteins bud off from TGN. These vesicles are called lysosomes. Later these vesicles fuse with vesicles which have arisen by pinacocytosis and phagocysis to form secondary lysosomes. Low pH of Lysosomes triggers the dissociation of enzymes from the receptors.
The M6P receptors are recycled back to trans-golgi network in vesicles. Lysosomes contain hydrolyzing proteolytic enzyme, which digests proteins meant for degradation. A protein named ubiquitin marks the proteins meant for destruction. Ubiquitin is present in all eukaryotic cells. This mechanism degrades only those proteins which are meant for destruction and not the proteins which are to be left alone.
The proteins meant for secretion travel to plasma membrane from trans-golgi network.
All this transportation of vesicles from the RER to the cis face of golgi to successive levels of golgi and on to their final destinations requires the high levels of specificity in targeting. Transport of vesicles to wrong destinations would lead to cellular chaos.
Targeting of Proteins to Mitochondria and Chloroplasts:
Mitochondria and chloroplasts possess their own DNA, ribosomes, mRNA and synthesize a few proteins. But most of the proteins required for mitochondria and chloroplasts are synthesized in cytosol by nuclear DNA and then imported into these organelles. Both these organelles are covered by double membranes. The proteins are translocated into these organelles after they are fully synthesized. This is known as post-translational translocation.
There are four mitochondrial locations where the proteins are targeted. These are outer membrane, inner membrane, intermembranal space and mitochondrial matrix. The proteins are released in unfolded state and they bind to a family of chaprones. These chaprones are cytosolic hsp 70 proteins (heat shock proteins) that deliver the proteins to an import receptor on the outer mitochondrial membrane.
The import receptor then slides to a site where inner and otuer mitochondrial membrane form a channel through which the unfolded protein enters into mitochondria leaving out cytosolic hsp 70 protiens. As the protein reaches the matrix, mitochondrial heat shock protein, mitochondrial hsp 70 binds to it. A protease cleaves the signal sequence.
These proteins have more than one successive N-terminal targeting signal sequence. The first signal sequence imports the protein into matrix and the second signal re-directs the protein into membranes or inter membranal space.
Mitochondria processes machinery for cellular respiration. Each membrane and each compartment of mitochondria has its unique proteins. Enzymes of electron transport chain lie in the inner membrane while most enzymes of citric acid cycle are found in the matrix.
Protein Targeting to Chloroplasts:
The newly synthesized proteins by free ribosomes are impored into chloroplasts as in mitochondria. Calvin cycle enzymes fix atmospheric CO2 into carbohydrates during photosynthesis.
Protein Targeting into Nucleus:
The nuclear envelope consists of outer and inner membranes and has inter membranous space between them. The outer membrane is continuous with ER and has ribosomes on it. Proteins for the nucleus are synthesized on free ribosomes in the cytosol and imported into nucleus through 3000-4000 nuclear pores known as nuclear pore complexes which are special gates.
The proteins that are imported into nucleus are in fully folded state and do not require any chaprones. Protiens imported into nucleus have targeting signal sequences on them which are called nuclear localization signals (NLS). Each one has 4-8 amino acids and they are internal sequences and not terminal. NLS is not cleaved from the protein. Due to this feature proteins can re-enter the nucleus whenever the nuclear envelope is lost during cell division.
Membrane Proteins:
The proteins embedded in different membranes may have single trans-membrane domain which is a segment of 20-25 amino acids. Other proteins may have many trans-membrane domains connected by loops on both sides of the membrane. These proteins are called multi-pass orientation proteins. In photosynthetic bacteria a protein called bacterio-rodospin spans 12-14 times across the lipid bilayer membrane of bacteria. It traps energy from sunlight and uses it to pump protons across the bacterial membrane.