Let us make an in-depth study of the urea cycle. After reading this article you will learn about: A. Steps of Urea Cycle:- 1. Formation of Carbamoyl Phosphate 2. Synthesis of Citrulline 3. Synthesis of Argininosuccinate 4. Cleavage of Argininosuccinate 5. Cleavage of Arginine and B. Metabolic Disorders of Urea Cycle:- 1. Hyperammonemia type-I 2. Hyperammonemia type-II 3. Citrullinemia 4. Arginino-succinic aciduria and 5. Hyper-argininemia.
A. Steps of Urea Cycle:
1. Formation of Carbamoyl Phosphate:
Condensation of ammonium ion with bicarbonate ion resulting in the formation of carbamoyl phosphate by the help of the enzyme carbamoyl phosphate synthase-I present in the liver mitochondria. It requires Mg2+ and a dicarboxylic acid i.e. N-acetyl glutamate. This step requires 2 ATPs.
2. Synthesis of Citrulline:
Carbamoyl phosphate formed in the first step combines with ornithine resulting in the synthesis of citrulline aided by the enzyme citrulline synthase or ornithine transcarbamoylase. Citrulline is easily permeable to the mitochondrial membrane and hence it diffuses into the cytosol.
3. Synthesis of Argininosuccinate:
In the cytosol, citrulline combines with the amino acid aspartate forming argininosuccinate catalysed by the enzyme argininosuccinate synthase. It requires ATP which is hydrolysed to AMP resulting in utilization of two high energy bonds. Mg2+ acts as cofactor.
4. Cleavage of Argininosuccinate:
The enzyme argininosuccinase acts reversibly to cleave argininosuccinate into Arginine and fumarate. Fumarate enters the TCA cycle (the linkage between TCA and urea cycle is known as Krebs bi-cycle).
5. Cleavage of Arginine:
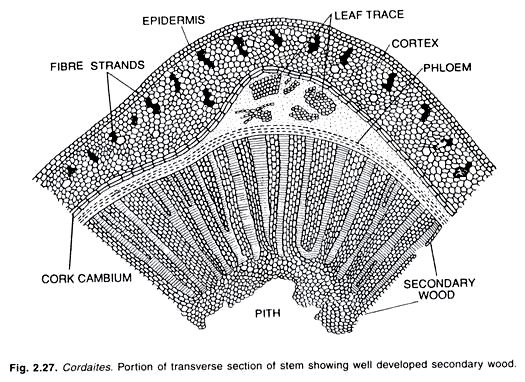
Arginine is lysed into ornithine and urea under the influence of the enzyme arginase. Hence arginine is known as semi-essential amino acid i.e. though it is synthesized in the body it is not available for protein synthesis. Ornithine is regenerated in this step and the urea cycle completes by the formation of urea. Ornithine and lysine are potent inhibitors of the enzyme arginase.
Arginase is also present in testis, renal tubules, mammary gland and skin in minute quantities. The intermediate amino acids formed in the urea cycle i.e. ornithine, citrulline and argininosuccinate are known as non-protein amino acids.
The overall equation of urea formation is:
NH4 + CO2 + Asparate + 3ATP → Urea + Fumarate + 2ADP + 2Pi + AMP + PPi
The urea cycle brings two amino groups and HCO3 together to form urea. Thus toxic, insoluble ammonia is converted into non-toxic, water soluble, excretable urea. Hence, urea cycle disposes two waste products i.e. NH4 and HCO3. This fact suggests that urea cycle participates in the regulation of blood pH, which depends on the HCO3/H2CO3. Though 3 ATPs are utilized, the ultimate cost of making a molecule of urea is 4 ATPs (one ATP is converted into AMP). The rate limiting steps of urea cycle are 1, 2, & 5.
B. Metabolic Disorders of Urea Cycle:
Since urea cycle converts toxic ammonia to urea, disorders of this cycle lead to ammonia intoxication. This ammonia intoxication is more when there is block at step 1 or 2. Common symptoms of the disorders of urea cycle are vomiting in infancy, avoidance of high protein diet, intermittent ataxia, irritability, lethargy and mental retardation.
1. Hyperammonemia type-I:
Due to the deficiency of carbamoyl phosphate synthase-I. It is a familial disorder.
2. Hyperammonemia type-II:
Due to the deficiency of ornithine transcarbamoylase. It is X-linked. Clinical finding is, the elevation of glutamine in the blood, CSF and urine.
3. Citrullinemia:
More citrulline is excreted in the urine i.e. upto 1 to 2 gm/day, due to the defect in the enzyme argininosuccinate synthase.
4. Arginino-succinic aciduria:
It is a rare recessive disease. Higher level of arginino-succinic acid in plasma and CSF. Usually present in the early age. Feeding arginine and benzoate promotes nitrogen excretion in these patients. This is due to lack of the enzyme argininosuccinase.
5. Hyper-argininemia:
High level of arginine due to lack of arginase enzyme.
Glucogenic and Ketogenic Amino Acids:
Those amino acids which on oxidation give intermediate compounds, resembling those of carbohydrate metabolism and which may be converted to glucose are termed as glucogenic amino acids. Ex. Ala, Arg, Asp, Asn, Cys, Gly, Glu, Gin, His, Pro, Met, Ser, Tyr, Val, Lys. Those amino acids which form acetate or acetoacetate intermediates found during fatty acid metabolism are termed as ketogenic amino acids i.e. they can give rise to ketone bodies. Ex. Leu. The amino acids lie, Lys, Phe, Tyr and Trp can give rise to both glucose and ketone bodies hence they are both glucogenic and ketogenic amino acids.
Oxidation of Carbon Skeleton of Amino Acids:
Once ammonia is released from the amino acids the remnant carbon back bone undergoes various oxidative reactions to yield one or the other intermediates of citric acid cycle (TCA cycle) as shown below—