The following points highlight the seventeen main applications of protoplasts technology. Some of the applications are: 1. Study of Osmotic Behaviour 2. Study of IAA Action 3. Study of Plasma-lemma 4. Study of Cell Wall Formation 5. Organelle Isolation 6. Study of Morphogenesis 7. Virus Uptake and Replication 8. Study of Photosynthesis from Isolated Protoplast and Others.
Protoplast Technology: Application # 1.
Study of Osmotic Behaviour:
Influence of different environmental factors on the osmotic behaviour can be studied using plant protoplasts.
Protoplast Technology: Application # 2.
Study of IAA Action:
When growth promoters like IAA are applied to plants, they act directly on plasma membrane of the cell and increase the permeability of the membrane to water resulting in cell elongation. This can be established by the use of protoplast in vitro.
When IAA is applied to the plasmolyticum containing protoplasts they expand rapidly and, finally, burst due to too much vacuolation (Cocking and Hall, 1974). Further, it can be verified by using anti-auxins that suppress this bursting, indicating that the site of action of IAA is the plasma lemma of the plant cell.
Protoplast Technology: Application # 3.
Study of Plasma-Lemma:
When newly released protoplasts are placed in hypotonic solution or plain water, the protoplasts burst within a second or if the protoplast are dropped from a certain height on a glass slide—the same result will happen. So by this process, plasma-membrane can be isolated very easily from protoplast and a number of study on plasma-membrane can be investigated.
Protoplast Technology: Application # 4.
Study of Cell Wall Formation:
The early deposition of cellulosic micro-fibril and their orientation at the protoplast surface can be followed using both light and electron microscope and has also provided much basic information concerning cell wall biology.
Protoplast Technology: Application # 5.
Organelle Isolation:
Protoplasts are very convenient material for the isolation of chloroplasts, mitochondria, nuclei and even chromosomes. It has been demonstrated that chloroplasts particularly isolated from cereal protoplast have higher capacity for CO2 fixation than those obtained by mechanical grinding.
Protoplast Technology: Application # 6.
Study of Morphogenesis:
Isolated protoplast provides an ideal single cell system. Under suitable condition, protoplast regenerates its own wall and becomes the walled cells. Cell division followed by plant regeneration may occur from such unique single cell system either through organogenesis or embryogenesis.
Plant regeneration is very important as well as significant for fusion experiment and for the experiment of genetic modification in protoplasts.
Protoplast Technology: Application # 7.
Virus Uptake and Replication:
The plant virus interrelationships in the past were not clearly known due to lack of suitable experimental systems that can easily infect the cells. But after the innovation of protoplast isolation and its culture, this problem is almost solved.
Protoplast can directly be inoculated with pathogenic virus in the medium. The process of uptake of virus particle, their replication inside the protoplasts and their mode of action at the molecular and cellular level are made possible by the aid of protoplasts.
Protoplast Technology: Application # 8.
Study of Photosynthesis from Isolated Protoplast:
Elegant experiments to investigate various biophysical and biochemical aspects of photosynthesis in C3 and C4 plants have been carried out by a number of workers using protoplasts.
Protoplast Technology: Application # 9.
Isolation of Bacteroids from Root Nodule Protoplast:
Viable bacteriods from root nodules of legumes has been isolated by first preparing nodule protoplast and then rupturing them either mechanically or by lowering suddenly the concentration of the plasmolyticum in the surrounding medium. This method ensures the freedom of the preparation of bacteria from the infected thread.
Protoplast Technology: Application # 10.
Genetic Engineering or Gene Transfer in Plant through Protoplast:
Since the isolated protoplast shows high picnocytic activity, so it can uptake some specific genes or foreign DNA and this property brings some genetic modification in plants.
Genetic modification by DNA uptake implies that DNA from one source be taken up and incorporated into the recipient genome and that genetic information encoded in the exogenous DNA be expressed as a new and stable characteristic in the recipient protoplast and subsequently in the regenerated plants. This approach provides an alternative way to increase genetic diversity to the existing genetic make-up of the plant.
Several approaches has been employed for transferring exogenous DNA or specific genes into the protoplasts such as:
i. Direct gene transfer.
ii. Indirect or liposome mediated delivery of DNA to plant protoplasts.
iii. Gene transfer using biological vectors.
iv. Electroporation.
v. Microinjection of DNA into protoplasts.
vi. Micro-projectiles and macro-injection.
vii. Fusion between the bacterial spheroplasts and plant protoplasts.
Direct uptake of foreign DNA has been investigated in protoplast. The anticipated steps in the uptake process into protoplasts include binding to specific sites on the plasma membrane and passage through the membrane and cytoplasm. This is followed by binding to specific binding sites, passage through the nuclear membrane into the nucleus and, ultimately, integration into the host genome (Fig. 6.9).
A major obstacle in feeding isolated DNA directly into the protoplasts is the presence of enzymes that degrade DNA. So it needs a good protection for exogenous DNA so that DNA makes a safe journey from external medium to the recipient nucleus and such protection will facilitate its stabilisation in recipient cytoplasm.
The use of liposome is a new innovation to facilitate the uptake of nucleic acid without any degradation. Liposomes are the liquid crystalline structure obtained when amphipathic lipids such as phospholipids are dispersed in water or aqueous salt solution.
Each liposome is a bilayered completely enclosed sac-like vesicle (250 A to 10 µm diameter). It is possible to enclose the nucleic acid within liposome which can readily transport through biological membrane of the protoplast. The result indicates that liposome can protect the enclosed nucleic acid from the degradation by the enzymes of the recipient protoplast.
The incorporation of DNA by vectors may also reduce the degradation of DNA and improve the gene transfer process.
Two types of vectors are being considered:
Plant viruses and
Bacterial plasmids.
Using the DNA virus as a vector, it may be possible to insert the foreign DNA into the viral genome. Since the foreign DNA in association with viral DNA can be incorporated safely in the nucleus of the recipient cell. So, DNA viruses are more or less suitable vectors for gene transfer.
Plasmids are double-stranded, closed circular extra-chromosomal DNA found in bacteria. Plasmids can integrate into the chromosome of the recipient cells. The research with plasmids has developed rapidly in the recent years and has resulted in new and revolutionary techniques for gene transfer.
On the other hand, a series of novel enzymes called restriction endonucleases have been discovered. Such enzymes could be used as ‘molecular scalpel’. The enzymes cut the DNA at points with specific nucleotide sequences. At the same time, ligase enzyme used as ‘molecular adhesive’ can join the cleaved DNA.
The restriction enzymes cleave the plasmid DNA to linear, double-stranded sections with overlapping and complimentary nucleotide sequences. The linear plasmid DNA section can be mixed with endonuclease-cleaved section of foreign DNA from other source. With the help of ligase, reconstituting the plasmid DNA carrying the genes or section of foreign DNA can be mixed with the protoplasts.
After the hybrid plasmid is taken up by the protoplast, it is replicated and the genetic information that is encoded in the foreign DNA can be transcribed and, eventually, expressed in the walled cells and subsequently in the plants derived from the protoplasts (Fig. 6.10).
It is well-known that the pathogenic bacteria Agrobacterium tumefaciens causes the plant tumour crown gall upon wounding and infection. The agents responsible for tumour induction are plasmid called tumour inducing or Ti plasmids.
Crown gall cells have their capacity to grow in vitro in absence of phytohormones. It has been established that the autonomous growth of the tumour cells is caused by the integration of a piece of DNA (T-DNA) form a Ti plasmid.
This naturally transformed system or Ti plasmid DNA can be used in experiments to transform plant protoplasts into hormone independent opine synthesizing cells. Thus, genetic manipulation of plant protoplast by means of Agrobacterium tumefaciens can be achieved by three different approaches:
(a) Fusion of crown gall protoplasts with normal protoplast,
(b) Infection of cell wall regenerating protoplast with virulent strains of A. tumefaciens,
(c) Transformation of protoplasts with Ti plasmid DNA.
With the help of above genetic engineering or plasmid technology, intensive efforts are being made in various laboratories, all over the world to transfer the ‘nif’ gene (nitrogen fixing gene) in the protoplast of non-legumes. It may well usher us into an era of “self-fertilised farming”.
Electroporation. Electric pulses have been shown to modify the structure of the plasma membrane, presumably by the formation of pore-like openings. This electrically-induced pore formation is known as electroporation.
It increases the permeability of the plasma membrane for certain period. This increased permeability has been exploited to introduce various macromolecules including DNA into freshly isolated plant protoplast. Indeed, electroporation is becoming a routine technique in both transient and stable gene expression studies.
Interestingly, electroporation also promotes DNA synthesis, cell division and colony formation in isolated protoplast. During electroporation, the pores of the plasma membrane appear to be around 30 nm in diameter and persist for several minutes after the pulse.
The electroporation pulse is produced by discharging a capacitor across the electrodes in a specially designed electroporation chamber (Fig. 6.11).
Exposure of protoplast suspension to electric fields can be done by two ways depending on the experimental material and the objective of the experiment:
(i) The application of the light voltage of short duration generated by a commercial machine, e.g., 1.5 KV cm-1 for 10 µ sec (microsecond) and
(ii) The application of a lower voltage pulse of much longer duration, e.g., 350 V cm-1 over 54 m sec (millisecond).
During the application of electric pulse, the protoplasts are suspended between the electrodes in an ionic solution containing alien DNA or engineered vector DNA. The protoplasts will be electroporated. Then the DNA molecules in the surrounding medium will enter the protoplast.
The transformed protoplasts are then plated out as normal and the colonies are selected after comparing with control one. The frequency of transformation of protoplast by electroporation is improved in presence of 13% PEG (polythelene glycol) if added after the DNA.
According to Shillito etal (1985) some other factors like linearizing the plasmid,’ use of carrier DNA, heat shocking (45°C, 5 min) and placing on ice after pulsing may all improve transformation frequency.
Under optimum conditions, transformation frequencies of up to 2% or greater have been reported. Stably transformed cell lines have been regenerated by electroporating in a number of monocotyledonous species including rice, wheat, maize. The regenerated plants are also fertile and free of somaclonal variations.
Apart from stable transformation, electroporation is used widely in transient gene expression for many purposes for example to study the properties of promoters or effects of modifications to DNA sequence that might modulate gene expression levels.
Protoplast Technology: Application # 11.
Microinjection of DNA to the Protoplast:
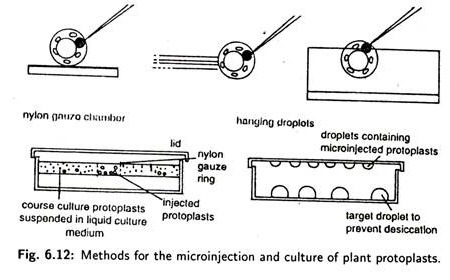
The microinjection technique is based on the use of glass micropipettes with a 0.5 to 10 µm diameter tip to effect the direct transfer of DNA into the cytoplasm or the nucleus of a recipient cell which is either immobilised on a solid support, artificially bound to a substrate or held by a pipette under suction.
For injection, plant cell (usually 1-2 days old protoplasts which have started to form a cell wall) are either immobilised on coverslips with poly-L-lysine or embedded in a thin layer of agarose.
An alternative immobilisation method involves the use of a specially designed ‘holding pipette’, (Fig. 6.12). In this method, a protoplast can be sucked up or released by developing positive or negative pressure in the holding pipette connected to a syringe.
The micropipette is first filled with the DNA solution and then directed into the immobilised target protoplast or cell with the aid of a micromanipulator. An inverted microscope equipped with differential interference optics is normally used to facilitate visualization of the nucleus.
When the micromanipulator is in the position within the nucleus, an appropriate volume of solution is injected by gentle air or hydraulic pressure exerted by a syringe connected to the micropipette at one end and to a pressure regulator system at the other end.
If cells are immobilised on coverslips or in agarose, then nuclei are usually inoculated after the cells have had time to recover, usually late on the first day after attachment or embedding. Specialised nurse culture or micro drop culture techniques are needed to recover microinjected cells (Fig. 6.12).
The plating efficiency after injection is usually reduced by anything from 10-70%. Southern blot analyses have shown that trans-formants resulting from microinjected cells have patterns of transferred DNA organisation similar to those found in other direct gene transfer systems.
Protoplast Technology: Application # 12.
Micro-Projectiles and Macro-Injection:
It is possible to deliver nucleic acids into plant protoplasts (usually 2-3 days old protoplasts which have already started to form a cell wall) on the surface of high velocity tungsten or gold micro-projectiles. The micro-projectiles are normally 1-3 µm in diameter and are accelerated into plant cells by either an explosive charge or using shock waves initiated by a high-voltage electric discharge (Fig. 6.13).
This technology basically involved loading tiny tungsten or gold spheres with DNA and then spreading the particles on the surface of a mobile plate or plastic bullet.
Then under a particle vacuum, the macro-projectile is fired against a retaining plate or mesh either by a shock wave caused by vaporizing a water droplet or by a cordite explosion; the macro-projectiles decelerates instantly, while the momentum and small size of the dense micro-projectile causes them to be thrown from the surface of the macro-projectile and to penetrate the target plant cells placed on a plate.
This technique is also popularly known as gene gun technique.
To date there have been several reports of transient gene expression following the micro- projectile bombardment. Using this technique, transformed tobacco plant have been recovered recently by several laboratories. But still considerable efforts are being made to improve this technique.
It is also possible to transform plant protoplasts by simply allowing them to soak up a solution of alien DNA. However, despite many attempts to repeat the procedure, now termed macro-injection, no further successful reports have been published.
Genetical change that occurs in bacteria due to absorption of foreign DNA or extract of other bacteria, is known as bacterial transformation. Transgenesis is a new term for the type of transformation in higher plant system.
Protoplast Technology: Application # 13.
Induction of Mutation and Genetic Variability:
It has been repeatedly observed that plant cell in culture show a wide range of genetic diversity. This phenomena can be exploited by plant breeders and geneticists for inducing variability in protoplast culture. The recessive characters can be detected in the regenerated plants derived from haploid protoplasts.
Therefore, haploid protoplast would make an ideal system for studying the effect of irradiation and for the induction of mutation by placing them in media supplemented with various chemical mutagens.
From this method, mutant line can be selected.
Protoplast Technology: Application # 14.
Microorganism Transplantation:
Incorporation of microorganisms like bacteria, blue-green algae, yeast etc. into protoplasts has been attempted with the immediate objective of establishing endosymbiotic association with higher plant cells which may eventually yield a plant having some beneficial activity.
Bacterial cell uptake by plant protoplasts has been investigated with species of Rhizobium and Spirillium. There are reports based on ultra- structural examinations that bacteria enter the cells by endocytosis and may become embedded in vesicles in the cytoplasm of protoplasts. Similar uptake studies were performed with yeast and blue-green algae cells.
Introduction of Anabaena varialis and nitrogen fixing blue- green algae Gleocapsa sp. into protoplasts has also been reported. However, nothing is known about the fate of the introduced microorganism, because there has been no reported evidence of survival or development of any organisms within the protoplasts.
Protoplast Technology: Application # 15.
Implantation of Chloroplast:
Plant protoplasts have ability to uptake the isolated chloroplasts by the process of endocytosis. Several reports have described uptake of chloroplasts. Chloroplasts isolated from Vaucheria dichotoma were implanted into carrot cell culture protoplasts.
The chloroplasts may enter the cytoplasm enclosed in membrane-bound vesicles, although the enclosing membrane in some cases is absent. Biological evidence of chloroplast gene expression was presented but the experiments have not been confirmed.
The inability and ability of chloroplasts to survive and multiply in recipient protoplasts have not been unequivocally demonstrated, although limited replication has been reported. Potentially the chloroplast uptake procedure offers an excellent approach to study chloroplast/cytoplasm and nuclear interrelationships, genetics and physiological autonomy and specificity of functions of the organelles.
Protoplast Technology: Application # 16.
Transplantation of Nuclei:
Isolated nuclei can be introduced into the protoplasts. Both intra and inter-specific nuclear transplantation have been observed in Petunia hybrida, Nicotiana tabacum and Zea mays. Retention, normal function or degradation of the incorporated nuclei is not known.
But it is really opening up new avenues for the study of nuclear-cytoplasmic interaction if fertile plants with foreign nuclei could be regenerated from such protoplasts.
Protoplast Technology: Application # 17.
Transplantation of Chromosome:
The uptake of isolated metaphase chromosomes has proven successful in plant protoplast. This procedure provide a valuable method for genetic information transfer and gene analysis.