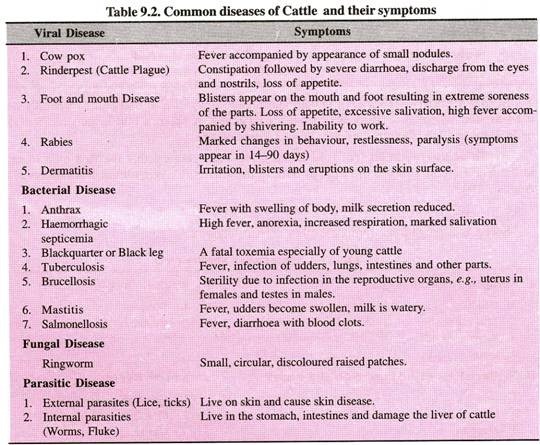
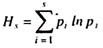In this article we will discuss about the organic and inorganic compounds of protoplasm.
Inorganic Compounds of Protoplasm:
The inorganic components of the protoplasm mostly occur in the form of water, minerals and salts which are as follows:
(i) Water:
The most abundant inorganic constituent of the protoplasm is the water. Water constitutes about 65 to 80 per cent of the protoplasm. In the protoplasm, the water occurs in two forms, viz., free water and bound water. The 95 per cent of the total cellular water is used by the protoplasm as the solvent for various inorganic substances and organic compounds and is known as free water.
The remaining 5 per cent of the total cellular water remains loosely linked with protein molecules by hydrogen bonds or other forces and is known as bound water.
The water contents of the cellular protoplasm of an organism depend directly on the age, habitat and metabolic activities. For instance, the cells of embryo have 90 to 95 per cent water which decreases progressively in the cells of the adult organism. The cells of lower aquatic animals contain comparatively high percentage of the water than the cells of higher terrestrial animals.
Further, the percentage of water in the protoplasm also varies from cell to cell according to the rate of the metabolism.
The water is the best biological solvent for inorganic substances such as mineral ions, salts, etc., and organic compounds such as carbohydrates and proteins. It forms the good dispersion medium for the colloidal system of the protoplasm. Because the water has high specific heat and high heat of vaporization, so it prevents drastic temperature changes in the cell. The temperature of cell remains relatively constant.
The water is a good conductor of the electricity therefore it dissociates various molecules which remain dissolved in it into ions. It has high surface tension. The water has greater importance for the various metabolic functions because most of them require exclusively the aqueous media.
The water is used by the cell as a transporting media for the food, nitrogen wastes and other necessary substances. The water is immiscible with the non-polar liquids as the lipids, so its molecules remain stable and unmixed with the lipid contents of the plasma membrane and other intra-cellular membranes.
(ii) Minerals:
The minerals are the inorganic chemical substances which occur in the crust of the earth. In the protoplasm the minerals usually occur in the form of salts and in combination with the organic compounds. The mineral salts occur in the form of ions in the protoplasm. An ion is an atom or group of atoms which carries one or more positive or negative charge.
The positively charged ions are known as cations and the negatively charged ions are known as anions. For example, when sodium chloride (NaCl) is dissolved in water, it is ionized to form a sodium cation (Na+) and a chlorine anion (CI–).
The inorganic compounds which by dissolving in water become ionized are known as electrolytes but those which do not dissociate in the solvent but remain as such in the molecular stated are known as non-electrolytes. The, protoplasm contains both electrolytes and non- electrolytes.
(a) Electrolytes:
The electrolytes play a vital role in the maintenance of osmotic pressure and acid base equilibrium in the protoplasm. Certain ions such as the magnesium (Mg+), etc., are essential for many enzymatic activities because these ions act as cofactor. The phosphate (PO4) ion is the important constituent of the adenosine triphosphate (ATP) which is the chief supplier of energy for most of the life processes. Other important ions of the protoplasm are sodium (Na+), potassium (K+), calcium (Ca+), chlorine (Cl–), carbonate (CO3), sulphate (SO2), and amino acids.
(b) Non-electrolytes:
Some of the minerals occur in protoplasm in non-ionising state. The non- electrolytes of the protoplasm are Na, K, Ca, Mg, Cu, I, Fe, Mn, Fl, Mo, CI, Zn, Co, Ni, etc. The iron (Fe) occurs in the haemoglobin, ferritin cytochromes and some enzymes as catalase and cytochrome oxidase.
The calcium (Ca) occurs in the blood, protoplasm and the bones. The copper (Cu), manganese (Mn), molybdenum (Mo) and zinc (Zn) are useful as cofactors for enzymatic actions. The iodine and fluorine are essential for the thyroid and the enamel metabolism respectively.
Organic Compounds of Protoplasm:
The chemical substances which contain carbon (C) in combination with one or more other elements as hydrogen (H), nitrogen (N), sulphur (S), etc., are called organic compounds. The organic compounds usually contain large molecules which are formed by the similar or dissimilar unit structures known as the monomers.
A monomer (Gr., mono = one; meros = part) is a simplest unit of the organic molecule which can exist freely. Some organic compounds such as carbohydrates occur in the protoplasm as the monomers.
The monomers usually link with other monomers to form oligomers (Gr., oligo = few or little; meros = part) and polymers (Gr., poly – many; meros =, part). The oligomers contain small numbers of monomers, while the polymers contain large number of monomers. The oligomers and polymers contain large-sized molecules or macromolecules.
When a polymer contains similar kinds of monomers in its macromolecule, it is known as homo-polymer and when the polymer is composed of different kinds of monomers, it is known as the heteropolymer. The main organic compounds of the protoplasm are the carbohydrates, lipids, proteins and nucleotides.
(i) Carbohydrates:
The carbohydrates (L., carbo-carbon or coal; Gr., hydro = water) are the compounds containing the carbon, hydrogen and oxygen. The carbohydrates form the main source of the energy for all living beings. Only green plants and certain microbes have the power of synthesizing the carbohydrates from the water and CO2 in the presence of sunlight and chlorophyll by the process of photosynthesis.
All the animals, non-green plants (e.g., fungi, bacteria and viruses) depend on green plants for the supply of carbohydrates.
Chemically the carbohydrates are the polyhydroxy aldehydes or ketones and they are classified as follows:
1. Monosaccharide’s (Monomers);
2. Oligosaccharides (Oligomers) and
3. Polysaccharides (Polymers).
1. Monosaccharide’s:
The monosaccharide’s are the simple sugars with empirical formula Cn (H2O)n.
They are classified and named according to the number of carbon atoms in their molecules as follows:
(i) Trioses contain three carbon atoms in their molecules, e.g., Glyceraldehyde and Dihydroxy acetone.
(ii) Tetroses contain four carbon atoms in their molecules, e.g., Erythrulose.
(iii) Pentoses contain five carbon atoms in their molecules, e.g., Ribose, Deoxyribose, Arabinose and Xylulose.
(iv) Hexoses contain six carbon atoms in their molecules, e.g., Glucose, Fructose and Galactose.
(v) Heptoses contain seven carbon atoms in their molecules, e.g., Sedoheptulose.
The monosaccharide’s are the monomers and cannot split further (or hydrolysed) into the simpler compounds.
The pentose’s and hexoses are the most abundantly found monosaccharide’s in the protoplasm. The pentose sugar, ribose is the important compound of ribonucleic acid (RNA) and certain coenzymes as nicotinamide adenine dinucleotide (NAD), NAD phosphate (NADP), adenosine triphosphate (ATP) and coenzyme A (CoA).
Another pentose sugar, the deoxyribose is the important constituent of the deoxyribonucleic acid (DNA). The ribulose is a pentose which is necessary for photosynthesis mechanism. The glucose, a hexose sugar is the primary source of the energy for the cell. The other important hexose sugars of the protoplasm are the fructose and galactose.
2. Oligosaccharides:
The oligosaccharides contain 2 to 10 monosaccharide’s (monomers) in their molecules. The monomers remain linked with each other by the glycosidic linkages.
Certain important oligosaccharides are as follows:
(i) Disaccharides contain two monomers, e.g., Sucrose, Maltose, Lactose, etc.
(ii) Trisaccharides contain three monomers, e.g., Raffinose, Mannotriose, Rabinose, Rhaminose, Gentianose and Melezitose.
(iii) Tetra saccharides contain four monomers, e.g., Stachyose and Scordose.
(iv) Pent saccharides contain five monomers, e.g., Verbascose.
The most abundant oligosaccharides are the disaccharides, viz., sucrose, maltose and lactose. The sucrose and maltose occur mainly in the protoplasm of plant cells, while lactose occurs exclusively in the protoplasm of animal cells. The molecules of sucrose are composed of D-glucose and D-fructose.
The molecules of maltose consist of D-glucose. The lactose is composed of two monomers, viz., D-glucose and D-galactose.
3. Polysaccharides:
The polysaccharides are composed of ten to many thousands monosaccharide’s as the monomers in their macromolecules. Their empirical formula is (C6H10O5)n. The molecules of the polysaccharides are of collodial size having high molecular weights. The polysaccharides can be hydrolysed into simpler sugars. The protoplasm contains two kinds of polysaccharides, viz., homopolysaccharides and heteropolysaccharides.
(i) Homopolysaccharides:
The homopolysaccharides have similar kinds of monosaccharide’s in their molecules. The most important homopolysaccharides of the protoplasm are the starch, glycogen and cellulose.
(a) Starch:
The molecules of the starch are composed of several units of amyloses and amylopectin’s. The starch is the storage food material of the plant cells.
(b) Glycogen:
The glycogen is a polymer of glucose molecules. It is the important storage food material of animal cells. The glycogen though occurs in most animal cells in smaller amount but the cells of molluscs, liver and muscles are rich in glycogen contents.
(c) Cellulose:
The cellulose is the polymer of the cellobiose (C12H22O11) which in turn contains many glucose molecules in its molecule. The cellulose forms the cell wall of the plant cells and provides mechanical support to the cell.
he plant cells beside starch and the cellulose contain other polysaccharides as xylan, alginic acids (algae), pectic acids, inulin, agar-agar and hemicellulose out of which some provide mechanical support, while others are used as stored food material.
(ii) Heteropolysaccharides:
The polysaccharides which are composed of different kinds of the monosaccharide’s and amino nitrogen or sulphuric or phosphoric acids are known as heteropolysaccharides.
The most important heteropolysaccharides are as follows:
(a) Neutral heteropolysaccharides:
The neutral heteropolysaccharides contain monosaccharide’s and the acetylated amino-nitrogen in their molecules and are known as acetyl glucosamines. The most important acetyl glucosamine of the animal cells is the chitin which is an important Constituent of the cells of the crustaceans and some insects. The chitin provides mechanical support to the cells.
(b) Acidic heteropolysaccharides:
The acidic heteropolysaccharides contain different kinds of monosaccharide’s and sulphuric or other acids in their molecules. The most important acidic heteropolysaccharides of the protoplasm of the animal cells are the hyaluronic acid, chondroitin sulphate and heparin.
The hyaluronic acid forms the cementing material of the connective tissues. It occurs in the skin, connective tissues and synovial fluid of the joints. The chondroitin sulphate occurs in the cells of the cartilage, skin, cornea, umbilical cord and it serves as a matrix for the bone formation. The heparin is a blood anticoagulant and is found in the liver, lung, thymus, spleen and blood.
(c) Mucoproteins and Glycoproteins:
When the acetyl glucosamines, monosaccharide’s and proteins unite together they form mucoproteins and glycoproteins. These include the blood group polysaccharides and occur in the red blood corpuscles, saliva, gastric mucin, ovomucoids, ovalbumins, serum and albumins, etc.
(ii) Lipids (Fats):
The lipids (Gr., lipos = fat) are the organic compounds which are insoluble in water but soluble in ether, chloroform, benzene, hot alcohol and petroleum ether. They contain long chains of aliphatic hydrocarbons or benzene ring in their molecules. The lipids are non-polar and hydrophobic. They are important constituents of the cellular membranes, hormones and vitamins of the cells and are the source of energy for the cells.
The lipids contain carbon (C), hydrogen (H) and oxygen (O) and are classified into following types:
1. Simple lipids:
Simple lipids are the esters of the alcohols or the triglycerides containing fatty acids and alcohols.
Triglyceride Lipase H2O
Glycerol + 3 Fatty acids.
The constituent fatty acids may be saturated, e.g., palmatic, stearic acids or unsaturated, e.g., oleic, linoleic, linolenic, arachedonic and clupanadonic acids. The lipids of the animal cell protoplasm contain the fatty acids, viz., palmatic, stearic, palmitoleic, oleic, linoleic and linolenic acids.
The simple lipids of the protoplasm are as follows:
(i) Natural fats:
The natural fats are the naturally occurring fats which occur in animal and plant cells as stored food substances.
(ii) Wax:
The waxes are the simple lipids and are the esters of fatty acids of high molecular weight with the alcohols except the glycerol. The most important constituent alcohol of the molecules of wax is the cholesterol, e.g., bees wax.
2. Compound lipids:
The compound lipids contain fatty acids, alcohols and other compounds as phosphorus, amino-nitrogen carbohydrates, etc., in their molecules.
The most important compound lipids of the protoplasm of animal and plant cells are as follows:
(i) Steroids:
These lipids contain a common cyclic nucleus of cyclopentano-perhydro- phenanthrene. The common steroids of the protoplasm of animal cells are the sex hormones, adrenocortical hormones, vitamin D and bile acids. The steroids which possess – OH group in their molecules are known as sterols.
The sterol alcohol cholesterol occurs in the bile, brain, adrenal gland, blood and other animal cells. The plant cells usually contain no cholesterol in their cellular protoplasm.
(ii) Phospholipids:
The phospholipids or phosphotids contain in their molecules, the glycerol, fatty acids and phosphoric acids.
The phospholipids are the main component of the lipids of the unit membranes of the cells. In animal cells, the phospholipids occur along with the choline, ethanolamine or serine. For instance, the phosphotidyl-cholines or lecithins occur in most animal cells and are important for the metabolism of fats in the liver cells.
The phosphotidyl ethanolamine and phosphatidyl serine are commonly called cephalins. The other important phospholipids of the matrix are the phosphoinositides (occur mostly in the cells of liver, brain, muscle and soyabean), plasmogen and isositides.
(iii) Sphingolipids:
The sphingolipids occur mostly in the cells of the brain and instead of the glycerol they contain in their molecules amine alcohol, sphingol or sphingosine. For instance, the mylein sheaths of the nerve fibres contain a lipid known as sphingomyelin which contains sphingosine and phosphatides in its molecules.
(iv) Glycolipids:
The glycolipids contain in their molecules the carbohydrates and the lipids. The protoplasm of the animal cells contain two kinds of glycolipids, viz., cerebrosides and gangliosides.
(a) Cerebrosides:
The cerebrosides contain in their molecules sphingosine, fatty acids and galactose or glucose. The cerebrosides are the important lipids of the white matter of the cells of brain and the myelin sheath of the nerve. The important cerebrosides are the kerasin, cerebron, nervon and oxynervons.
(b) Gangliosides:
The gangliosides have complex molecules which are composed of sphingosine, fatty acids and one or more molecules of glucose, lactose, galactosamine and neuraminic acid. The gangliosides occur in the grey matter of the brain, membrane of erythrocytes and cells of the spleen.
(v) Lipoproteins:
The lipoproteins contain lipids and proteins in their molecules and occur in blood of mammals in association with plasma proteins. In lipoproteins, cholesterol and α and β globulins are found.
(vi) Carotenoids:
The carotenoids are the compound lipids and they form pigments of the animal and plant cells. There are about 70 carotenoids occurring in both types of cells. The important carotenoids of cells are the α β and ![]() carotenes, retinene, xanthophylls, lactoflavin in milk, riboflavin (vitamin B2), xanthocyanins, coenzyme Q, anthocyanin flavones, flavonols and flavonones, etc. Chemically carotenoids are porphyrins. The porphyrin (Gr., porphyra = purple) are the carotenoids (a lipid).
carotenes, retinene, xanthophylls, lactoflavin in milk, riboflavin (vitamin B2), xanthocyanins, coenzyme Q, anthocyanin flavones, flavonols and flavonones, etc. Chemically carotenoids are porphyrins. The porphyrin (Gr., porphyra = purple) are the carotenoids (a lipid).
These are the cyclic compounds formed by four pyrrole (CH4NH) rings linked by methylene bridges. The porphyrins linked with metals and proteins forming the important pigments of animal and plant cells as the chlorophyll and haemoglobin. The chlorophyll is a porphyrin containing magnesium. Many pigments of animal cells such as haemoglobin, myoglobin and cytochromes contain porphyrins in their molecules.
(iii) Proteins:
The proteins (Gr., proteno = occupy first place) are the most important constituents of the protoplasm. All proteins are composed of carbon (C), hydrogen (H), oxygen (O), nitrogen (N) and some of them in addition contain sulphur (S) and phosphorus (P). The protoplasm is dependent almost entirely upon proteins for its supply of nitrogen, sulphur and phosphorus.
The proteins are the polymers of the amino acids. An organic compound containing one or more amino groups (-NH2) and one or more carboxyl groups (-COOH) is known as amino acid. The amino acids occur freely in the protoplasm and constitute the so called amino acid pool. The amino acids are derived from the organic acids in which the hydrogen in alpha position is replaced by the amino groups.
For example, glycine is formed by the acetic acid and alanine derived from propionic acid as shown by following reactions:
Here the NH2 is the amino group, -COOH is the carboxylic group, while the ‘R’ represents variety of chemical combinations which occur in different types of amino acids.
The protoplasm contains about 22 amino acids, e.g., glycine, alanine, valine, leucine, isoleucine, glutamic acid, aspartic acid, arginine, lysine, threonine, serine, cysteine, cystine, methionine, phenyl alanine, tyrosine, tryptophan, proline, hydroxy proline, histidine, glutamine and aspargine, etc.
Formation of proteins:
Because molecule of the amino acid contains both amino (-NH2) and acidic or carboxyl (-COOH) group, it can behave as an acid and base at a time. The molecules of such organic compounds which contain both acidic and basic properties are known as amphoteric molecules. Due to amphoteric molecules, the amino acids unite with one another to form complex and large protein molecules.
When two molecules of amino acids are combined, then the basic group (-NH2) of one amino acid molecule combines with the carboxylic (-COOH) group of other amino acid and the loss of a water molecule takes place. This sort of condensation of two amino acid molecules by -NH-CO linkage or bond is known peptide linkage or peptide bond.
A combination of two amino acids by the peptide bond is known as dipeptide, when three amino acids are united by two peptide bonds they form tripeptide.
Likewise, by condensation of few or many amino acids by the peptide bonds, the oligopeptides and polypeptides are formed respectively. Various molecules of polypeptides unite to form the peptones, proteases and proteins. Thus, protein macromolecules are the polymers of many amino acid monomers. The macromolecules of proteins comparatively have high molecular weights.
Properties of the proteins:
The proteins are amphoteric compounds and, therefore, act as acids and bases. The solubility of proteins depends on the pH value of the solvent. The proteins can bind salts by forming ionic bonds. The proteins are precipitated by salts of heavy metals and by high temperature. Certain proteins are specific for a particular species and they possess antigen properties. Proteins coagulate on heating.
Uses of proteins:
The functional proteins or enzymes function as specific catalysts in most biosynthetic and metabolic activities of the cell and the structural proteins form various structures of the cell.
(iv) Enzymes:
The protoplasm and many cellular organelles contain very important organic compounds known as the enzymes (Gr., en = in+zyme = leaven). The enzymes are the specialised proteins having the capacity to act as catalysts in chemical reactions.
Like other catalysts of the chemical world, the enzymes are the catalysts of the biological world and they influence the rate of a chemical reaction, while themselves remain quite unchanged at the end of the reaction. The substance on which the enzymes act is known as substrate.
The enzymes play a vital role in various metabolic and biosynthetic activities of the cell such as synthesis of DNA, RNA and protein molecules and metabolism of carbohydrates, lipids, fats and other chemical substances.
The enzymes of the protoplasm and cellular organelles are classified as follows:
1. Oxireductases:
The enzymes catalysing the oxidation and reduction reactions of the cell are known as oxireductases. These enzymes transfer the electrons and hydrogen ions from the substrates, e.g., hydrogenases or reductases, oxidases, oxygenases and peroxidases.
2. Transferases:
The enzymes which transfer following groups from one molecule to other are known as transferases one carbon, aldehydic or ketonic residues, acyl, glycosyl, alkyl, nitrogenous, phosphorus containing groups and sulphur containing groups.
3. Hydrolases:
These enzymes hydrolyse a complex molecule into two compounds by adding the element of water across the bond which is cleaved. These enzymes act on the following bonds: ester, glycosyl, ether, peptide, other C-N bonds, acid anhydride, C-C, halide and P-N bonds. Certain important hydrolase enzymes are the proteases, esterases, phosphatases, nucleases and phosphorylates.
4. Lysases:
The lysases enzymes add or remove group to or from the chemical compounds containing the double bonds. The lysases act on C-C, C-O, C-N, C-S and C- halide bonds.
5. Isomerases:
These enzymes catalyse the reaction involving in the isomerisation or intra-molecular rearrangements in the substrates, e.g., intra-molecular oxidoreductases, intra-molecular transferases, intra-molecular lysases, cis-trans-isomerases, racemerases and epimerases.
6. Ligases or Synthetases:
These enzymes catalyse the linkage of two molecules by splitting a phosphate bond. The synthetases enzymes form C-O, C-S, C-N and C-C bonds.
Classification of Enzymes on the Basis of Chemical Nature of Substrate:
According to the chemical nature of the substrate, the enzymes have been classified as follows:
1. Carbohydrases,
2. Proteases (endopeptidases and exopeptidases),
3. Amidases,
4. Esterases,
5. Dehydrogenases,
6. Oxidases,
7. Decarboxylases,
8. Hydrases,
9. Transferases,
10. Isomerases.
The enzymes are specific in action and many factors such as pH, temperature and concentration of the substrate effect the rate of the activity of enzymes. Certain enzymes, viz., zymogens occur in the inactive form and these are activated by other enzymes known as kinases to perform catalytic activities.
For instance, the enzyme trypsinogen of the pancreatic cells is activated in the intestine by the enzyme enterokinases and the enzyme pepsinogen of the Chief cells of the stomach is activated by the hydrochloric acid which is secreted by parietal cells.
Prosthetic groups and coenzymes:
Certain enzymes such as cytochromes are the conjugated proteins and contain prosthetic groups as metalloporphyrins complex in their molecules. Certain enzymes cannot function singly but they can function only by the addition with the small molecules of other chemical substances which are known as coenzymes. The inactive enzyme (which cannot function singly) is known as the apoenzyme.
The apoenzyme and coenzyme are collectively known as holoenzyme. For instance, the enzyme hydrogenase is an apoenzyme which can function either with the coenzyme NAD+ or NADP.
Some important coenzymes or cofactors are nicotinamide adenine dinucleotide (NAD) or diphosphopyridine nucleotide (DPN), nicotinamide adenine dinucleotide phosphate (NADP) or triphosphopyridine nucleotide (TPN), flavin adenine mononucleotide (FMN), flavin adenine dinucleotide (FAD), ubiquinone (coenzyme Q), lipoic acid (LIP or S2), adenosine triphosphate (ATP), pyridoxyl phosphate (PALP), tetrahydrofolic acid (COF), adenosylmethionine, biotin, coenzyme A (CoA), thiamine pyrophosphate (TPP) and uridine diphosphate (UDP).
Isoenzymes:
Recently it has been investigated that some enzymes have similar activities and almost similar molecular structures. These enzymes are known as isoenzymes. The isoenzymes in the cell, e.g., lactic dehydrogenase (LDH) occur in five identical isoenzymes.
(v) Vitamins:
The vitamins are complex organic compounds of diverse chemical nature which are required in minute amounts for normal growth, functioning and reproduction of cells. The vitamins play an important role in the cellular metabolism and they act as the enzymes or other biological catalysts in the various chemical activities of the cell.
Their importance for the animal has been reported by Hopkins, Osborne, Mendel and McCollum (1912-1913). Funk (1912) demonstrated the presence of basic nitrogen in them and gave the name vitamins, i.e., vital amines to them. The cell cannot synthesise the vitamins from the standard food and so they are taken along with the food. Their deficiency in the cell causes metabolic disorders and leads to various diseases.
The vitamins of utmost biological importance have been tabulated in the following table:
Hormones:
Hormones are the complex organic compounds which occur in traces in the protoplasm and regulate the synthesis of mRNA, enzymes and various other intracellular physiological activities. The most important hormones are growth hormone, estrogen, androgen, insulin, thyroxine, cortisone, adrenocortical hormones, etc.
These hormones are synthesised by the endocrine glands and transported to various cells of multicellular organisms. In cells they regulate various metabolic activities. For example, the ecdysone hormone has been found to form puffs (Balbiani rings) in the giant chromosomes of insects.
In mammalian liver cells, the enzymes which convert glucose into glycogen are regulated by the hormone insulin which is synthesised by the islets of Langerhans in the pancreas. Moreover, the hormone thyroxine, secretion of thyroid gland, activates the enzyme phosphorylase to form glucose phosphate from glycogen.
Adrenalin produced by adrenal medulla maintains the tone of involuntary muscles. Pituitary gland produces many important hormones, prolactin starts and maintains secretions of milk, inter-median controls expansion of pigments and oxytocin helps in child birth.
Gonads produce androgens and estrogens which control the development of sex organs and secondary sexual characters. Hormones have been studied largely in vertebrates, but in the head of insects is a pair of small endocrine glands called corpora allata which produce hormones which control metamorphosis, moulting and egg production.
Nucleic Acids:
The nucleic acids are the complex macromolecular compounds of immense biological importance. They control the important biosynthetic activities of the cell and carry hereditary information’s from generation to generation.
There occur two types of nucleic acids in living organisms, viz., ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Both types of nucleic acids are the polymers of nucleotides. A nucleotide is composed of nucleoside and phosphoric acid.
Even the nucleoside is composed of the pentose sugar (ribose or deoxyribose) and nitrogen bases (purines and pyrimidine’s). The purines are adenine and guanine and pyrimidine’s are cytosine, thymine and uracil.
Deoxyribonucleic acid (DNA):
Deoxyribonucleic acid (DNA) molecule was first of all isolated by F. Miescher in 1869 from the nuclei of pus cells, spermatozoa and red blood cells of the birds. DNA is found only in the nucleus from where it controls and guides the activities of a cell.
DNA is a compound of very high molecular weight (over one million), it has a giant molecule made of smaller molecules linked together, but its molecular weight is variable. The DNA molecule consists of a pentose sugar called deoxyribose, and phosphoric acid to which are joined four bases of pyrimidine’s and purines.
The bases of purine are adenine (A) and guanine (G), while those of pyrimidine are cytosine (C) and Thymine (T). The bases are always paired, G is always paired with C in equal quantities, and A is always paired with T in equal amounts, these pairs are tied to each other by hydrogen bonds.
When a sugar molecule is linked to a phosphate and a pyrimidine or purine base is attached to this sugar, then this new molecule made of three parts is called nucleotide. A nucleotide is a single structural unit of nucleic acid; it is made of pentose sugar, a nitrogenous base, and phosphoric acid. Only four kinds of nucleotides are possible in DNA, because there are four kinds of bases (A, C, G and T).
All four kinds of nucleotides are very similar and they differ only in the kind of purine or pyrimidine base they contain. But a DNA molecule is made of a very large number of nucleotides linked together serially forming two chains.
The sugar and phosphate constituents of nucleic acid hardly vary, but the four bases of purine and pyrimidine show great variety in their arrangement, sequence, and possible variations. The ratio of the four bases differs widely in different organisms, but there are always equal numbers of cytosine and guanine, and also equal numbers of adenine and thymine.
In 1953, Watson and Crick presented a model of a DNA molecule, according to their model, all the units or nucleotides of the long DNA molecule are joined by phosphates and arranged in two single chains wound together in a coiled helix and cross-linked between the bases, thus, the molecule is in the form of a spiral ladder.
Each upright part of the ladder is made of a series of phosphates and sugars, and the cross-linked rungs of the ladder are the base pairs of purines and pyrimidine’s linked together by hydrogen; each rung has either an adenine paired with thymine or cytosine paired with guanine.
A molecule of DNA may have 20,000 such purine-pyrimidine base pairs. The two chains of a DNA molecules are complementary and each chain is capable of enormous variety due to the numbers and arrangement of base pairs. The two chains may form thousands of coils of the helix. The sequence of base pairs along the two chains represents coded information.
The quantity of DNA in a nucleus is constant in nearly all conditions. DNA never leaves the nucleus, it directs the activities of a cell from the nucleus. It has the power of self-duplication by splitting longitudinally into two chains along its hydrogen bonds, after separation each of the two chains builds another exactly like its partner from which it has separated.
Thus, it can make a copy of itself due to which a cell divides into two. DNA molecules have the unique property of adhering to each other forming an orderly structural pile. When these molecules come together they influence other molecules in their environment to arrange themselves in a template or mould so that an order is introduced among smaller organic molecules.
Ribonucleic acid (RNA):
Ribonucleic acid (RNA) has the same constituents as DNA except that it contains ribose sugar which has one more atom of oxygen than in deoxyribose sugar of DNA, and its bases are adenine, cytosine, guanine and uracil (its pyrimidine base is uracil in place of thymine). There are four kinds of nucleotides in RNA because there are only four bases (A, C, G and U).
According to present evidence, RNA is made of a single chain of nucleotide rather than the double chain of DNA. RNA is first formed by DNA in the nucleus where it may be stored in the nucleolus, but most of it passes out into the cytoplasm either directly or after being stored in the nucleolus.
It lies within particles which either float freely in the cytoplasm or line the membranes in a cell, it is active in ribosomes and microsomes.
Some RNA is believed to be a messenger (mRNA) carrying information from DNA to the sites of protein synthesis in the cytoplasm, while some other kinds of RNA pick up particular kinds of amino acids to form the molecules of proteins. The quantity of RNA varies with the nutritional conditions of a cell.
Nucleic acids play a major role in various protoplasmic activities and together with proteins they form the foundation of all living phenomena, they transmit instructions from one cell to another, they also bring about cell division, they form the core of viruses because a particle of any virus is composed of a protein linked intimately with DNA, and studies on nucleic acids have shown how viruses work.
DNA controls and guides the synthesis of proteins in the cytoplasm through various types of RNA in association with ribosomes.
In this synthesis of proteins, the DNA in the nucleus controls the formation of specific RNA, in other words the DNA serves as a template or mould for synthesis of RNA. This is known as messenger RNA (mRNA) which passes out through the pores of the nuclear membrane into the cytoplasm and becomes associated with ribosomes, then this messenger RNA becomes a template.
Other kinds of RNA called transfer RNA (tRNA) which are already present in the cytoplasm now pick up individual amino acids and take them to the ribosomes. Various transfer RNAs along with their amino acids get aligned on the RNA of ribosomes in a specific order to form a new polypeptide chain which is a part of a protein molecule.
This process is repeated until the synthesis of a protein is completed. The kind of protein produced is determined by its particular sequence of amino acids. The transfer RNA molecules are now freed and each becomes available to again pick up an amino acid of a particular kind. Such a control of nucleic acids over synthesis of proteins is an indirect control over all the chemical reactions of a cell.
Some proteins are used directly to form parts of a cell, while other proteins function as enzymes which control the types of chemical reactions that can take place in cells. Many enzymes also contain nucleic acids, such as the energy-carrier adenosine triphosphate (ATP) which is present in all living organisms and plays the role of storing and supplying energy.
Nucleic acids control the genes of a chromosome which are made of DNA.
The genes are responsible for determination of hereditary characters. The DNA content of a cell is constant and it passes intact from the parent cells to the daughter cells. In mitosis the DNA content becomes doubled so that the daughter cells receive DNA identical to that of parent cells.
In a cell, the DNA may be dispersed or condensed with proteins to form nucleoproteins, the nucleoproteins then are the components of chromosomes and genes.
The DNA content is directly connected with chromosomes and genes, that is with the hereditary content of cell.
The chromatin of chromosomes resolves itself into four major molecules, they are histone, a protamine, DNA and RNA, but DNA is the key molecule because it is the chemical basis of heredity and one molecule of DNA may be the seat of many genes, each gene serves as the source of hereditary information.
DNA is the main part of hereditary genes, in fact some authorities believe that each gene is a single molecule of nucleoprotein.
The genes pass exact replicas of themselves to all cells descending from the original zygote, secondly the genes control each and every step of development of an organism, thus, DNA controls and guides heredity and development.