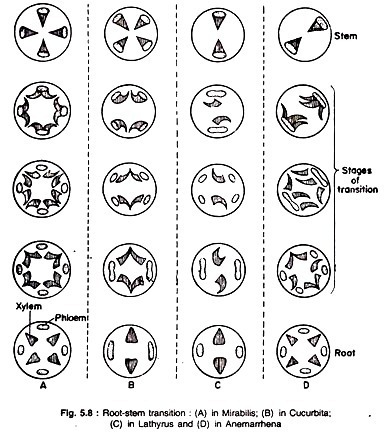
This article throws light upon the three main types of pharmacokinetic variables of drugs. The variables are: 1. Plasma Half-Life (t1/2β) 2. Volumes of Distribution 3. Total Body Clearance (CIB).
Contents
Type # 1. Plasma Half-Life (t1/2β):
Half-life is defined as the time of declining the initial plasma drug concentration by half. It is also known as the half-time of elimination or elimination half-time and is extremely important. It is regarded as the standard value in biological metabolism, i.e. for the same drug in the same animal under the same conditions, it is always the same.
Sulfonamides, for example are classified on the basis of their rate of excretion into short acting (t1/2β up to 7 hr), medium acting (t1/2β from 7 to 16 hr) and long acting (t1/2β > 16 hr).
First Order Half-Life:
The t1/2β for the first order reaction may be calculated by means of the following equation:
For this equation, it is obvious that for a first order reaction t1/2β is a constant. No matter what the initial concentration of a drug is, the time required for the amount to decline by one-half is a constant.
Uses of Half-Life:
(i) As a guide to the time taken for drug to be eliminated from the body.
(ii) As a guide to the rate of accumulation of drug in the body during multiple dosing.
(iii) As a guide to the relationship between the loading dose and the maintenance dose.
Rate Constants:
If a drug is administered directly into blood stream it is simultaneously distributed as well as eliminated. A decrease in blood concentration is described by the kinetics of transport to parallel compartments. Some of the drugs give biphasic disposition curve if plasma concentrations are plotted against time after i.v. administration.
where K12 = rate constant for drug transfer from central compartment, K21 = rate constant for drug transfer from peripheral to central compartment, K2 = rate constant for elimination from the central compartment.
The concept of K2 and β must be clear because both are different, β is the overall rate constant for elimination from the body. K2 is also designated as Kel or Ko.
Type # 2. Volumes of Distribution:
Volume of distribution has been defined as the volume of body fluids which holds the drug in solution at the same concentrations as the plasma. It is an important parameter that relates plasma concentration to the amount of drug in the body. In multi-compartment kinetics one can consider mathematically calculated volumes of central compartment and volume of the peripheral compartment.
There are several volume of distribution that may be considered for a drug that assumes a two-compartment open model e.g. volume of central compartment (VdC), apparent volume of distribution at steady state (Vdss), extrapolated volume of distribution (VdB) and volume of distribution by area (Vdarea). The calculations for each of these volumes are shown below.
i. Volume of the Central Compartment (VdC):
The volume of the central compartment (VdC) is useful for describing changes in drug concentration; since the central compartment is usually the sampling compartment. VdC is useful in the determination of drug clearance. In addition, the magnitude of VdC gives an indication of the distribution of the drug in body water. VdC is determined by dividing the dose (mg/kg) with the instantaneous plasma drug concentration (C0P).
Example:
A cow was given sulfadimethoxine i.v. at the dose of 214 mg/kg. The C0p (A + B) calculated after plotting the data on semi-log graph was 43 mg%. The Vdc calculated will be 50% of body weight.
At zero time (t=0) all of the drug in the body is in the central compartment. C0p is equal to A + B because at zero time, when t = 0, e0 = 1. Therefore,
Cp0 = Ae–α,0 + B–β.0
= Ae0 + Be0 = A + B
ii. Apparent Volume of Distribution at Steady State (VdC):
At steady state conditions the rate of the drug entry into the tissue compartment from the central compartment is equal to the rate of drug exit from the tissue compartment into the central compartment. The Vdss is calculated as follow;
Thus, Vdss may be considered as a function of the transfer constants K12 and K21.
iii. Extrapolated Volume of Distribution (Vdexp or VdB):
Vdexp is calculated by the following equation:
Vdexp may also be calculated by the following formula:
The above equation shows that any change in the drug distribution that can bring change in the value of VdC can also bring change in the value of Vdexp.
Sometimes the apparent volume of distribution can be related to a known identifiable fluid volume of the animals body, more often, the apparent volume of distribution is greater than even that of the total body water in different species of animals.
The theoretical volume simply gives an index of how widely or extensively the drug is distributed to the tissue of the body compared with the plasma. The drugs having higher Vd value are more extensively distributed in the body tissues.
If drug is highly bound to plasma protein its Vd value will be low because it will be less distributed to the body tissues. The Vd value of a drug must be roughly proportional to the circulating non protein bound fraction.
Type # 3. Total Body Clearance (CIB):
It is the fraction of the apparent volume of distribution from which the drug is removed in unit time. It is expressed in units of amount per time (mlmin-1), and sometimes it is corrected for body weight of the animal (mlkg-1 min-1).
For example, if the clearance of nor floxacin is 5 mlmin-1 in a goat with an apparent volume of distribution, of 6 liter, then 5 ml of the 6 litres is cleared of drug per minute. It can be said that it is the another way of expressing the rate constants for loss of drugs.
It is calculated by the following formula:
Using the previous example of nor-floxacin suppose that the plasma norfloxacin concentration is 50µg/ml and 5 mlmin-1 of 6 liters (Vd) is cleared of the drug per minute. Therefore, the rate of drug removal is equal to 5 mlmin-1 50µgml-1 i.e. 250 µgmin-1. Thus, 250µg of nor-floxacin is eliminated every minute from the body of goat when the plasma concentration is at 50 µgml-1.
Thus, the ClB value may be used to estimate the rate of drug elimination at any drug concentration. The given example for norfloxacin in goat provides a means for calculating clearance. If the clearance rate of norfloxacin is 250µg min-1, which can be measured by urinary excretion and that the plasma norfloxacin concentration is 50µgml-1, at this time, its clearance can be calculated as next page:
Total body clearance is equal to the sum of the clearance by all routes of elimination and usually divided into renal and non-renal clearances.
Renal Clearance (CIR):
It is the fraction of the apparent volume of distribution from which the drug is eliminated from the animals body by renal excretion in unit time. Thus it is the product of multiplication of fraction of the drug in the body excreted in urine (in unit time) and volume of the central compartment.
Where, Ae is amount of drug excreted in urine
Hepatic Clearance (CLH):
The above definitions for clearance value may also be employed here to define the hepatic clearance as that fraction of the apparent volume of distribution from which drug is removed by metabolism and biliary excretion in unit time. Similar to renal clearance the following equation is derived for the hepatic clearance.
ClH = Drug excreted in bile + metabolite in venous blood/Plasma drug concentration
However, for drugs whose non-renal clearance is entirely by the hepatic route (and that is the case for most drugs), the problems can be solved by measured total clearance and renal clearance.
Hepatic clearance will be the difference between the two:
The apparent first order rate constant for absorption (Ka) from depot, may be a complex constant. In case of a solid dosage form it can be affected not only by the case with which the compound itself is absorbed from the depot, but also be such factor as the rate of tablet disintegration and rate of dissolution of the solid.
If a pro-drug, is administered, Ka for the appearance of active drug in the blood may also reflex hydrolysis of the pro-drug. It is impossible to compare amount of drug absorbed or the ratio of the drug in tissue to plasma by comparing blood level curves for two different drugs, even when administered through same route to the same animal.