This article provides a close look on metabolism of amino acids.
Proteins are the most abundant organic compounds and constitute a major part of the body dry weight (10-12 kg in adults). They perform a wide variety of static (structural) and dynamic (enzymes, hormones, clotting factors, receptors etc.) functions.
About half of the body protein (predominantly collagen) is present in the supportive tissue (skeleton and connective) while the other half is intracellular.
The proteins on degradation (proteolysis) release individual amino acids. Amino acids are not just the structural components of proteins. Each of the 20 naturally occurring amino acids undergoes its own metabolism and performs specific functions. Some of the amino acids also serve as precursors for the synthesis of many biologically important compounds (e.g. melanin, serotonin, creatine etc.), Protein metabolism is more appropriately learnt as metabolism of amino acids.
Contents
Metabolism of Amino Acids — General Aspects:
The amino acids undergo certain common reactions like transamination followed by deamination for the liberation of ammonia. The amino group of the amino acids is utilized for the formation of urea which is an excretory end product of protein metabolism.
The carbon skeleton of the amino acids is first converted to keto acids (by transamination) which meet one or more of the following fates:
1. Utilized to generate energy.
2. Used for the synthesis of glucose.
3. Diverted for the formation of fat or ketone bodies.
4. Involved in the production of non-essential amino acids.
A general picture of amino acid metabolism is depicted in Fig. 67.13.
Transamination:
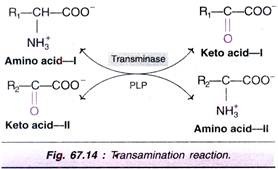
The transfer of an amino (~NH2) group from an amino acid to a keto acid is known as transamination (Fig. 67.14). This process involves the inter-conversion of a pair of amino acids and a pair of keto acids, catalysed by a group of enzymes called transaminases (recently, aminotransferases).
The salient features of transamination are:
1. All transaminases require pyridoxal phosphate (PLP), a coenzyme derived from vitamin B6.
2. There is no free NH3 liberated; only the transfer of amino group occurs.
3. Transamination is reversible.
4. It involves both catabolism (degradation) and anabolism (synthesis) of amino acids. Transamination is ultimately responsible for the synthesis of non-essential amino acids.
5. Transamination diverts the excess amino acids towards energy generation.
6. The amino acids undergo transamination to finally concentrate nitrogen in glutamate. Clutamate is the only amino acid that undergoes oxidative deamination to a significant extent to liberate free N3 for urea synthesis.
7. All amino acids except lysine, threonine, proline and hydroxyproline participate in transamination.
Deamination:
The removal of amino group from the amino acids as NH3 is deamination. It results in the liberation of ammonia for urea synthesis. Deamination may be either oxidative or non- oxidative.
Urea Cycle:
Urea is the end product of protein metabolism (amino acid metabolism). The nitrogen of amino acids converted to ammonia is toxic to the body. It is converted to urea and detoxified. As such, urea accounts for 80-90% of the nitrogen containing substances excreted in urine.
Urea is synthesized in liver and transported to kidneys for excretion in urine. Urea cycle is the first metabolic cycle that was elucidated by Hans Krebs and Kurt Henseleit (1932), hence it is known as Krebs-Henseleit cycle. The individual reactions, however, were described in more detail later on by Ratner and Cohen.
Urea has two amino (—NH2) groups, one derived from NH3 and the other from aspartate. Carbon atom is supplied by CO2. Urea synthesis is a five-step cyclic process, with five distinct enzymes. The first two enzymes are present in mitochondria while the rest are localized in cytosol. The reactions of urea cycle are depicted in Fig. 67.15.
Metabolism of Individual Amino Acids:
The metabolisms of certain individual amino acids are very briefly given in the form of overviews.
Glycine:
Glycine (Gly, G) is a non-essential, optically inactive and glycogenic (precursor for glucose) amino acid. It is indispensable for chicks. The outline of glycine metabolism is depicted in Fig. 67.16. Glycine is actively involved in the synthesis of many specialized products (heme, purines, creatine etc.) in the body, besides its incorporation into proteins, synthesis of serine and glucose and participation in one-carbon metabolism.
Phenylalanine and Tyrosine:
Phenylalanine (Phe, F) and tyrosine (Tyr, Y) are structurally related aromatic amino acids. Phenylalanine is an essential amino acid while tyrosine is non-essential. Besides its incorporation into proteins, the only function of phenylalanine is its conversion to tyrosine. For this reason, ingestion of tyrosine can reduce the dietary requirement of phenylalanine. This phenomenon is referred to as ‘sparing action’ of tyrosine on phenylalanine.
The predominant metabolism of phenylalanine occurs through tyrosine. Tyrosine is incorporated into proteins and is involved in the synthesis of a variety of biologically important compounds—epinephrine, norepinephrine, dopamine (catecholamine’s), thyroid hormones—and the pigment melanin (Fig. 67.17).
During the course of degradation, phenylalanine and tyrosine are converted to metabolites which can serve as precursors for the synthesis of glucose and fat. Hence, these amino acids are both glucogenic and keto-genic.
Tryptophan:
Tryptophan (Trp, W) was the first to be identified as an essential amino acid. It contains an indole ring and chemically it is α-amino β-indole propionic acid. Tryptophan is both glucogenic and keto-genic in nature. It is a precursor for the synthesis of important compounds, namely NAD+ and MADP+ (coenzymes of niacin), serotonin and melatonin (Fig. 67.18).
Sulfur Amino Acids:
The sulfur-containing amino acids are methionine, cysteine and cystine. Among these, only methionine is essential. It serves as a precursor for the synthesis of cysteine and cystine which are, therefore, non-essential. An overview of the metabolism of the sulfur amino acids is depicted in Fig. 67.19.
Glutamate and Glutamine:
Glutamate and glutamine are non-essential glycogenic amino acids. Both of them play a predominant role in the amino acid metabolism and are directly involved in the final transfer of amino group for urea synthesis. In Fig. 67.20, an outline of glutamate and glutamine metabolism is given.
Fate of Carbon Skeleton of Amino Acids:
After the removal of amino groups, the carbon skeleton of amino acids is converted to intermediates of TCA cycle or their precursors.
The carbon skeleton finally has one or more of the following fates:
1. Oxidation via TCA cycle to produce energy (about 10-15% of body needs).
2. Synthesis of glucose.
3. Formation of lipids—fatty acids and ketone bodies.
4. Synthesis of non-essential amino acids.
The carbon skeletons of the 20 standard amino acids (or the amino acids of proteins) are degraded to one of the following seven products—pyruvate, α-ketoglutarate, succinyl CoA, fumarate, oxaloacetate, acetyl CoA and acetoacetate.
Some authors use the term amphibolic (Greek: amphiboles— uncertain) intermediates to these compounds due to their multiple metabolic functions. The amino acids are classified into three groups, based on the nature of the metabolic end products of carbon skeleton (Table 67.1).
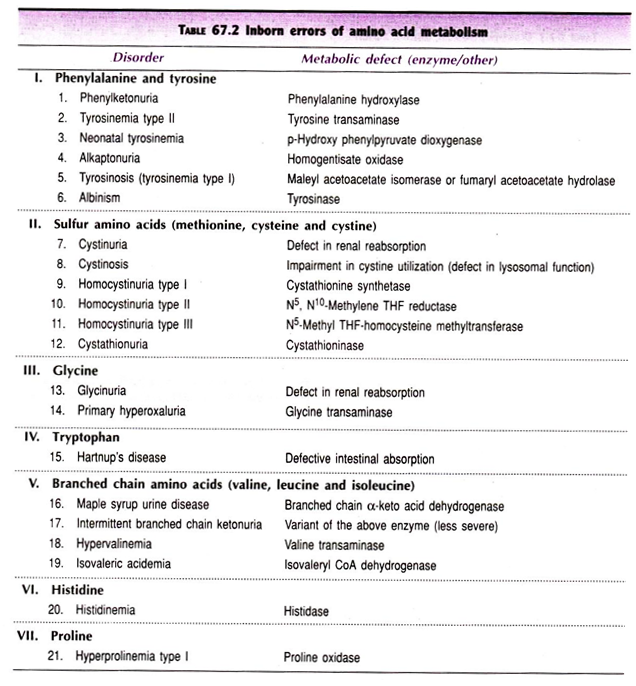
Inborn Errors of Amino Acid Metabolism—A Summary:
Several inherited disorders are associated with amino acid metabolism. In Table 67.2, a summary of major diseases and the enzyme defects is given.