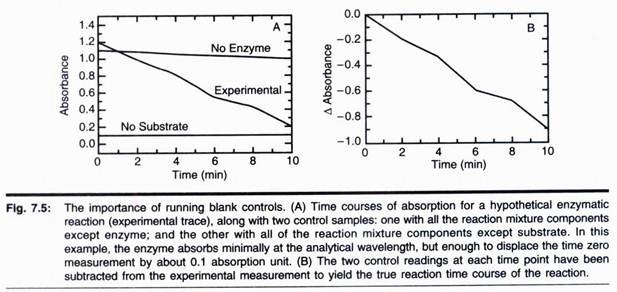
In this article we will discuss about Balanoglossus:- 1. Habit and Habitat of Balanoglossus 2. External Morphology of Balanglossus 3. Body Wall 4. Coelom 5. Endoskeleton 6. Digestive System 7. Respiratory System 8. Blood Vascular System 9. Excretory System 10. Nervous System and Sense Organs 11. Reproductive System 12. Development 13. Affinities.
Contents:
- Habit and Habitat of Balanoglossus
- External Morphology of Balanglossus
- Body Wall of Balanglossus
- Coelom of Balanglossus
- Endoskeleton of Balanglossus
- Digestive System of Balanglossus
- Respiratory System of Balanglossus
- Blood Vascular System of Balanglossus
- Excretory System of Balanglossus
- Nervous System and Sense Organs of Balanglossus
- Reproductive System of Balanglossus
- Development of Balanglossus
- Affinities of Balanglossus
Contents
- 1. Habit and Habitat of Balanoglossus:
- 2. External Morphology of Balanglossus:
- 3. Body Wall of Balanoglossus:
- 4. Coelom in Balanoglossus:
- 5. Endoskeleton in Balanoglossus:
- 6. Digestive System of Balanoglossus:
- 7. Respiratory System of Balanoglossus:
- 8. Blood Vascular System of Balanoglossus:
- 9. Excretory System of Balanoglossus:
- 10. Nervous System and Sense Organs of Balanoglossus:
- 11. Reproductive System of Balanoglossus:
- 12 Development of Balanoglossus:
- 13. Affinities of Balanoglossus:
1. Habit and Habitat of Balanoglossus:
Balanoglossus is a burrowing and exclusively marine animal. It is found in shallow waters between tide marks along the coast of warm and temperate oceans. Balanoglossus is world-wide in distribution. Balanoglossus is tubicolous living in U-shaped burrows excavated in the sandy bottom. The walls of the tube are lined with mucus secreted by the mucous gland of the animal.
The burrows are open at both ends, and spiral coils of faeces like the castings of earthworms may be seen at the posterior opening. In its burrow Balanoglossus lies in a twisted condition but its anterior and posterior extremities are straight. Knight Jones (1952) reported that the animal moves in its burrow with the help of cilia present all over the body.
2. External Morphology of Balanglossus:
The body of Balanoglossus is soft, elongated, cylindrical, being richly ciliated all over and covered with mucus.
The length of animal varies from 2 cm to 2.5 meters. Most forms are drab coloured, though reddish tints are present, several species are luminescent due to mucus. They have an offensive odour. The body is bilaterally symmetrical and divided into three regions, viz., proboscis or protosome, collar or mesosome, and trunk or metasome.
Proboscis:
The proboscis forms the anterior part of the body and is either rounded or conical in shape. It is continued posteriorly into a short, narrow neck or proboscis stalk.
The proboscis is hollow and has thick muscullar walls. Its cavity opens to the outside by means of a small opening called the proboscis-pore. In certain cases there are two proboscis-pores. In some species the proboscis-pore does not communicate with the proboscis-coelom, but terminate blindly, and may send off a narrow tubular diverticulum which opens into the neurocoel.
The proboscis sits in the collar somewhat like an acorn in its cup, a character that has given the name “acorn worms” to the group. The mouth, which is always wide open and incapable of closing completely, lies on the ventral side and its lips are the ventral edges of the collar region.
Collar:
The collar lies posterior to the proboscis and anterior to the trunk. It is a short cylinder usually about as wide as long and mostly shorter than the proboscis although sometimes longer. The funnel-like anterior part of the collar, the collarette, embraces the proboscis stalk and usually also the posterior part of the proboscis. Posteriorly the collar is sharply demarcated from the trunk by a circular indentation.
The surface of the collar is often marked with elevations, depressions, and specially circular grooves. The collar is also muscular and possesses two coelomic cavities. The right and left coelomic cavities are separated from one another by dorsal and ventral mesenteries. The coelomic cavities of collar are completely cut off from the proboscis cavity.
The collar cavity as well as the proboscis cavity are crossed by numerous strands of connective tissue which give the region a spongy appearance. The collar cavity communicated with the exterior by a pair of collar-pores, and short ciliated tubes (canals) leading into the first gill pouches.
The functional significance of the cavities and water pores in the proboscis and collar may best be explained through a description of the burrowing habits. When on the surface of the sandy bottom Balanoglossus pushes the tip of the proboscis into the sand, moving it around by muscular contractions until a shallow, cylindrical hole is made.
Then the proboscis empties its water content through its pore and collapses. This allows the collar to enter the hole. By taking in water through the pores the collar expands so as to fit lightly into the hole like a cork in a bottle. The well-filled collar then gives a point of resistance for further rooting movements of the refilled proboscis, which loosens sand and stows it into the scoop-shovel mouth.
Then both proboscis and collar relax and the latter squirms deeper into the hole before tightening its hold again. Once the collar gets a firm grip, the animal makes rapid progress and soon buries itself. The tail end is left near the surface, and at intervals comes out and deposits a pile of castings somewhat after the fashion of earthworms.
Trunk:
The trunk is the elongated posterior part of the body. It is somewhat flat and annulated on the surface. It has a mid-dorsal and a mid-ventral longitudinal ridge. The trunk is divisible into three parts, an anterior branchio-genital region, a middle hepatic region, and a posterior abdominal or post-hepatic region.
On the dorsal of the branchio-genital region of the truck is a double row of small pores the branchial apertures. Each row is situated in a long furrow. These pores increase in number during growth. In some species the most anterior are overlapped by a posterior prolongation of the collar called the operculum.
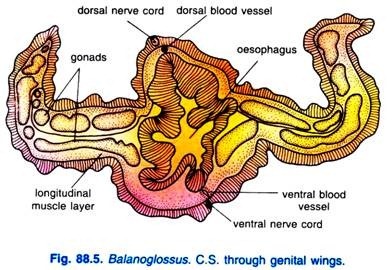
A pair of longitudinal genital ridges or genital wings extends throughout a considerable part of the body behind and in the region of the branchial apertures. In these genital ridges, gonads are situated. In some genera, the genital ridges are so prominent that they form a pair of wing-like lateral folds, the genital wings, but in other genera folds are absent.
The hepatic region is marked externally with irregular elevations due to sacculations produced by projecting hepatic caeca of the intestine. The abdominal region is longest and cylindrical. It tapers gradually and has a terminal anus. The coelom of the trunk is divided into two lateral closed cavities by vertical partition.
3. Body Wall of Balanoglossus:
The body wall of Balanoglossus is made up of an outer epidermis and an inner musculature.
1. Epidermis:
It consists of a single layer of epithelial cells. The epithelial cells are of tall columnar type and have their nuclei near their broader bases.
These cells are mainly of two types:
(i) Ciliated epidermal cells are more numerous and each bears cilia at its free end;
(ii) Gland cells are lying interspersed between the ciliated epidermal cells and are further of three kinds
(a) Goblet cells are flask-shaped and secrete mucus;
(b) Reticulate cells are long cells with vacuolated cytoplasm which also secrete mucus;
(c) Mulberry cells are long cells containing coarse cytoplasmic granules and, hence, are also called granular gland cells.
They secrete amylase. The mucus, secreted by gland cells, covers the animal and lines its burrow. The mucus has an obnoxious smell. In addition to these cells, the body wall of proboscis and anterior part of the collar also contain neurosensory cells which take darker stain than the rest. There is no dermis.
Immediately below the epidermis is a thick nervous layer consisting of bipolar and quadripolar nerve cells and fibres which form a network lying in close contact with the epidermal cells. This layer is traversed by the filamentous bases of the epidermal cells that are connected with the basement membrane.
The fibres of sensory epidermal cells synapse with the fibres of nerve cells. Below the nervous layer is a thick basement membrane made up of two lamellae pressed together. The basement membrane supports the epidermis and serves for attachment of underlying muscles.
2. Musculature:
The musculature of typical body-wall and gut-wall is greatly reduced and more or less replaced by muscles arising from the coelomic epithelium. The muscle fibres are smooth and of circular, longitudinal and diagonal types. The muscle layer lies below the basement membrane.
The proboscis musculature comprises a thin layer of circular muscle fibres and a thick layer of longitudinal muscle fibres. The longitudinal muscles fibres obliterate the proboscis coelom and some of the fibres cross one another diagonally. The collar musculature is confined to the collarette and consists of an inconspicuous layer of circular fibres and prominent bands of longitudinal and diagonal fibres.
The longitudinal and diagonal fibres, along with connective tissue, also traverse the collar coelom in a criss-cross pattern. The trunk musculature consists mainly of moderately developed longitudinal muscle fibres which are better developed on the ventral side. The muscle layer is interrupted by the dorsal and ventral mesenteries and the lateral septa.
Several radial muscle fibres are also found in the trunk region. The radial muscle fibres extend between the digestive tract and the body wall and traverse the trunk coelom.
4. Coelom in Balanoglossus:
The coelom is enterocoelous having been formed as outgrowths of the enteron. Corresponding with the three body regions the coelom is divided into three portions which are completely separated from each other by septa. The coelom is lined with coelomic epithelium or peritoneum.
But enteropneusts are peculiar in that their coelomic epithelium has connective tissue and muscle fibres which fill much of the original coelomic cavities, and a distinct peritoneal lining has disappeared, moreover the coelomic musculatre largely replaces the body wall muscles. The three parts of the coelom are an unpaired proboscis coelom, a pair of collar coeloms, and a pair of trunk coeloms.
1. Proboscis Coelom:
The proboscis coelom or protocoel is a single space in the proboscis which is largely occupied by muscles and connective tissue and a few structures like buccal diverticulum, glomerulus and central sinus or heart.
Dorsally, towards the posterior side, the proboscis coelom is divided by a dorsal mesentery into right and left dorsolateral compartments which extend into the proboscis stalk; the left compartment is larger than the right and communicates with the exterior through the proboscis pore situated mid-dorsally at the base of the posterior stalk.
Ventrally the proboscis coelom is divided by a ventral mesentery into right and left ventrolateral compartments which are continuous behind the mesentery.
2. Collar Coelom:
The collar coelom or mesocoel has two cavities lying side by side in the collar, one on each side between the collar wall and buccal cavity. The two cavities are partitioned by incomplete mid-dorsal and mid-ventral mesenteries. The collar coelom does not communicate with the proboscis coelom, but posteriorly, its each cavity opens into the first gill sac of its side by a canal called collar canal.
Each collar coelom opens to the exterior by a collar pore. The collar coelom is greatly obliterated by the collar musculature and connective tissue.
3. Trunk Coelom:
The trunk coelom or metacoel has two closed cavities lying between the body wall and alimentary canal. The two cavities are separated by an incomplete dorsal and a complete ventral mesentery.
In the branchiogenital region each cavity is further divided by a lateral septum into a dorsolateral and ventrolateral compartment. The trunk coelom is separated from the collar coelom by a collar-trunk septum. The trunk coelom is obliterated by the trunk musculature.
Coelomic Fluid:
The proboscis and collar coeloms communicate with the exterior and get filled with sea water through their pores, which keeps them turgid. The trunk coelom is filled with a watery coelomic fluid having amoeboid coelomocytes. The coelomocytes originate from the coelomic epithelium.
Each coelomocyte possesses a single large vacuole. According to Spengel, they behave like leucocytes by secreting a membrane around any foreign body that may invade the animal.
5. Endoskeleton in Balanoglossus:
In Balanoglossus there is no definite endoskeleton but there are four structures of a supporting nature, they are buccal diverticulum, proboscis skeleton, branchial skeleton, and a pygochord.
(i) Buccal Diverticulum:
Buccal diverticulum is a hollow preoral outgrowth, extending from the roof of the buccal cavity into the proboscis. It was for a long time also called a notochord or a stomochord on the assumption that it represents the anterior portion of the notochord of chordates. The buccal diverticulum extends forward in some enteropneusts as a slender vermiform process or appendix.
The buccal diverticulum is neither analogous nor homologous with the chordate notochord, histologically it is identical with the wall of the buccal cavity, it is apparently nothing more than a pre-oral extension of the gut.
(ii) Proboscis Skeleton:
Proboscis skeleton or nuchal skeleton is formed by the basement membrane becoming thick to form a laminated plate from which arise two thin horns or cornua, the plate usually has a mid-ventral keel. The proboscis skeleton lies in the proboscis stalk, while its horns extend into the roof of the buccal cavity.
(iii) Branchial Skeleton:
The walls of the U-shaped gill-clefts are supported by skeletal rods called primary and secondary gill-rods formed by thickening of the basement membrane.
(iv) Pygochord:
Pygochord is longitudinal rod-like structure extending from the ventral side of the intestine to the body wall, its function is not known but it may support the soft abdominal region.
6. Digestive System of Balanoglossus:
Alimentary Canal of Balangolossus:
In Balanoglossus, the alimentary canal is a straight tube. Its anterior opening, the mouth, is wide and circular and situated on the ventral side in a groove between the proboscis stalk and collarette. The mouth remains open constantly.
The posterior opening or the anus is a circular aperture at the extreme posterior end of the trunk. Between the mouth and anus, the alimentary canal can be distinguished into four regions buccal tube, pharynx, oesophagus, and intestine. Their walls are composed of ciliated epithelium lined externally by basement membrane and devoid of muscle fibres.
1. Buccal Tube:
The mouth leads into a buccal tube or cavity in the collar region. Its epithelial wall contains glandular goblet cells. The dorsal wall of buccal tube forms a short, stiff and hollow buccal diverticulum that projects into the proboscis coelom. It extends up to the collar-trunk septum behind which it continues into the pharynx.
2. Pharynx:
The wall of the roof of the buccal tube opens into the pharynx lying in the branchial region of the trunk. Its wall bears a longitudinal constriction along each lateral side.
These lateral constrictions project into its lumen as ridges, called parabranchial ridges consisting of tall columnar cells. These ridges and constrictions incompletely divide the pharynx into a dorsal branchial portion (pore pharynx) and a ventral digestive portion (digestive pharynx).
(i) Branchial Portion of Pharynx:
The dorsal branchial portion of pharynx is perforated dorsolaterally by two rows of U-shaped gill-slits. It is concerned with respiration.
(ii) Digestive Portion of Pharynx:
The digestive portion of pharynx is concerned with the food concentration, digestion and absorption of food. Its ciliated epithelial wall contains gland cells.
3. Oesophagus:
Behind the last pair of gill-slits the pharynx continues into the oesophagus. The dorsal and ventral divisions of the pharynx continue for some distance into the oesophagus; in this region, the dorsal part of the oesophagus is called post-branchial canal which possesses thick, folded and glandular epithelium. The posterior part of the oesophagus reduce in diameter and has deeply furrowed epithelium.
4. Intestine:
Behind the oesophagus is an intestine, It occupies the hepatic and post-hepatic regions of the trunk. The hepatic region of the intestine is highly vascular. Its epithelial cells are dark green or dark brown and its dorsal wall forms numerous prominent sacculations called hepatic caeca which push the body wall outwards and are, thus, visible externally.
The post- hepatic region of the intestine is connected with the ventral body wall by the pygochord. The intestine has the form of a simple tube and bears a pair of dorsolateral grooves lined by tall epithelial cells bearing long cilia. The intestine opens out through the anus situated at the extreme hind end of the body. The anus often possesses sphincter muscles.
Food, Feeding and Digestion of Balangolossus:
Balanoglossus is a “ciliary feeder”. Its food comprises microscopic organisms and minute organic particles present in water and mud or bottom sand in which it makes its burrows. The lateral cilia lining the gill-slits set up a current of water directed backward which enters through the mouth, takes its course through the buccal tube, pharynx, gill-slits and branchial sacs, and leaves through gill-pores.
This is called respiratory-cum-food current. Some food particles directly enter the mouth with this current, while some come in contact with the proboscis and are entangled in the mucus that covers it.
The mucus is secreted by the gland cells of the proboscis epithelium. Cilia covering the proboscis direct the mucous string, containing food particles towards the preoral ciliary organ at the base of the proboscis.
From here the mucous string is passed back into the mouth by the action of the proboscis cilia, assisted by the main water current entering the mouth. Organic particles present in the mud or sand are ingested directly along with mud or sand at the time of burrowing.
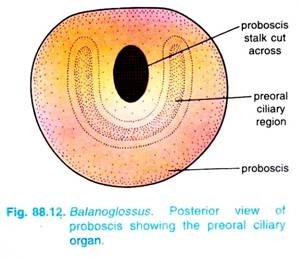
At the base of the proboscis, on the ventral side, there is a U-shaped depression bordered by long epidermal cells bearing long cilia. This structure is called the pre-oral ciliary organ.
It tests the quality of food and the water entering the mouth. Undesirable substances are prevented from entering the mouth by the ventral part of the collarette which does so by covering the mouth. Thus, the rejected particles, instead of entering the mouth, pass back over the collar.
Backward movement of food through the alimentary canal is maintained by the cilia lining its walls. In the pharynx, the food passes through the ventral digestive portion.
The exact process of digestion in Balanoglossus is not known with certainty; however, the digestion of food is brought about by the enzymes secreted by proboscis, gland cells of the pharynx, oesophagus and hepatic region of the intestine. Proboscis secretes mucus, which contains amylase, is ingested with the food.
The gland cells of pharynx and oesophagus also secrete enzymes. It is also claimed that hepatic caeca secrete amylase, maltase, lipase and weak protease. The enzymes digest the organic particles in the mud or sand. Undigested substances, along with mud or sand, pass out through the anus in large quantities which forms piles of “castings” at the posterior opening of the burrow.
7. Respiratory System of Balanoglossus:
The respiratory organs of Balanoglossus comprise:
(1) The branchial portion of pharynx bearing gill-slits
(2) The branchial sacs that open out through gill-pores
1. Branchial Pharynx:
As already described, two lateral longitudinal parabranchial ridges divide the pharyngeal cavity into a dorsal respiratory or branchial portion and a ventral digestive portion. Dorsolaterally, on each side, the branchial portion of pharynx is perforated by a longitudinal series of numerous U-shaped openings, the gill- slits. The number of gill-slits varies and increases as the animal grows older.
Each gill-slit is a broad oval slit in the beginning, but later, a hollow projection of dorsal pharyngeal wall, called tongue bar, grows into the slit making it U-shaped. The hollow tongue bars enclose coelomic cavity and do not touch the ventral side of the gill-slits. The portions of the pharyngeal wall between two adjacent U-shaped gill-slits are called gill-septa. The gill-septa are solid and do not enclose coelom.
A tongue bar is connected with its adjacent gill-septa by longitudinal connections are called synapticula. The tongue-bars and gill- septa are supported by M-shaped skeletal rods.
The middle arm of an M-shaped rod is bifurcated at the free end and lies in a gill-septum, while its outer arms lie in adjacent tongue bars. Thus, each tongue bar contains two arms of two adjacent skeletal rods. Each U-shaped gill-slit is richly lined by cilia, called lateral cilia.
2. Branchial Sacs:
Gill-slits do not open directly to outside. Each gill-slit opens into a gill- pouch called branchial sac which lies between the body wall and the pharynx. Each branchial sac
in turn opens to the exterior by a small, independent gill-pore.
However, in Balanoglossus misakiensis the first four branchial sacs become united to open by a common gill-pore to outside. The collar coelom also communicates with the common branchial sac of its side through a collar canal. The gill-pores are visible externally in two longitudinal rows, one on each side of the mid- dorsal ridge in the branchiogenital region of the trunk.
Mechanism of Respiration:
The lateral cilia lining the gill-slits create a current of water (food-cum-respiratory current) that enters the pharynx through mouth, then passes through gill-slits into the branchial sacs and finally leaves through the gill-pores. The tongue bars are richly supplied with blood capillaries and take part in respiration. The blood of their capillary networks takes up oxygen dissolved in water and diffuses carbon dioxide to it.
8. Blood Vascular System of Balanoglossus:
The blood vascular system of Balanoglossus consists of closed vessels, lacunar spaces and a definite pulsating organ, generally known as the heart. The blood is colourless and has no or very few corpuscles; it may contain a few detached endothelial cells; it has no respiratory pigment. Most of the blood vascular system is located between the lamellae of the basement membrane and the leaves of the mesentery.
There are two main longitudinal vessels, the dorsal and ventral vessels, running along the length of the body. The dorsal vessel is situated just below the dorsal nerve cord and above the alimentary canal and runs through the dorsal mesentery. The blood flows anteriorly through the dorsal vessel.
he ventral vessel is located in the ventral mesentery and the blood flows posteriorly in it. These two vessels are highly contractile and their walls are composed of an inner endothelium surrounded by muscle layer.
The dorsal vessel extends from the anus to the collar where it occupies a median position between two perihaemal cavities. The dorsal vessel is dilated at the front of the collar, forming a venous sinus which passes anteriorly into a central sinus or heart. The central sinus is situated above the buccal diverticulum. Immediately above the central sinus there is a triangular contractile epidermal sac called heart vesicle.
Blood from the central sinus enters the glomerular sinuses of the proboscis complex. In glomerulus the blood is cleared of nitrogenous wastes. From the glomerulus, the blood is collected by four vessels. These vessels are regarded as the arteries because the blood leaving the glomerulus is considered to be purified.
These arteries are:
(i) A mid-dorsal proboscis artery,
(ii) A mid-ventral proboscis artery and two efferent glomerular arteries. The mid-dorsal and mid-ventral proboscis arteries supply blood to the proboscis.
The efferent glomerular arteries run backward along the two sides of the buccal diverticulum. These vessels then run ventrally to encircle the buccal tube as the peribuccal arteries. The peribuccal arteries unite together ventrally to form a single longitudinal ventral vessel.
The ventral vessel runs up to the posterior end of the body through the ventral mesentery. On its way, the ventral vessel gives out a ventral collar vessel to supply the collar. The collar tissue contains two distinct lacunar networks which communicate posteriorly with a ring vessel. The ring vessel is located in the collar- trunk septum. It arises from the ventral vessel and is connected with the dorsal vessel.
A lateral pharyngeal vessel appears in the lacunar plexus at the junction of the two pharyngeal regions. The ventral vessel continues up to the anus and gives off lacunar networks all along the alimentary canal. The ventral vessel gives out an afferent branchial artery to each gill-septum which bifurcates to supply the two adjacent tongue-bars.
Thus, each tongue-bar receives two afferent branchial arteries which break up into a plexus. From this plexus an efferent branchial vein is formed. It runs dorsally up to the middle of the tongue- bar and joins with the efferent branchial vein of the adjacent tongue-bar. The common branchial vein opens into the dorsal vessel (Fig. 88.17).
The ventral vessel supplies blood through lacunar plexus to the body wall and alimentary canal. The blood from the intestinal plexus in the trunk region is collected mostly by the dorsal vessel.
9. Excretory System of Balanoglossus:
In Balanoglossus, the excretory organ is glomerulus or proboscis gland lying in front of the central sinus and projecting into the proboscis coelom. The glomerulus is made up of several blind tubular projections formed by the peritoneum covering the buccal diverticulum, central sinus and heart vesicle. The tubular projections of glomerulus are filled with blood which is confluent with the blood of the central sinus.
The covering of the glomerulus is composed of excretory peritoneal cells. The excretory peritoneal cells of glomerulus contain yellow or brown granules, probably of excretory substances. The excretory waste substances from the glomerulus pass on into the proboscis coelom from where they finally pass out to the exterior through the proboscis pore.
10. Nervous System and Sense Organs of Balanoglossus:
The nervous system of Balanoglossus is of a very primitive type resembling that of coelenterates and echinoderms. Nervous system consists primarily of an epidermal plexus or a layer of nerve cells and nerve fibres lies just below the epidermis. Threadlike processes of the epidermal cells contribute to the network or nerve net.
The nervous layer is composed of longitudinal nerve fibres with bipolar and multipolar nerve cells at the margin.
The nervous layer becomes thickened along definite strands to form two main nerve cords, one mid-dorsal and the other mid-ventral, which run along the entire length of the trunk. Ventral cord extends up to collar-trunk septum where it is connected with the dorsal cord by a circular strand, called circumenteric nerve ring.
Dorsal cord extends anteriorly up to the base of proboscis where it is connected with another circular strand called anterior nerve ring. From the anterior nerve ring longitudinal nerve fibres are given out, these nerve fibres are called subsidiary longitudinal cords of proboscis. In the collar region, dorsal cord leaves the epidermis and projects into the collar coelom as the collar cord.
The collar cord contains a cavity called neurocoel. The collar cord arises from the epidermis, but has sunk in to take a deeper position. Its similarity to chordate nerve cord, formed by the invagination of the nervous ectoderm at the dorsal mid-line, is evident. The collar cord and sometimes the anterior part of the dorsal cord in the trunk or nerve ring, contains giant neurons.
Each gives off a single large nerve fibre which crosses to the other side of the body and runs through the circumenteric ring to the ventral nerve cord.
The number of giant neurons varies from about 10 to 160. They are responsible for rapid conduction of stimuli leading to quick retraction of body parts. Although the collar cord is the most complex part of the nervous system, it is no more than a conduction path and the site of giant neuron formation.
Sense Organs of Balanoglossus:
In Balanoglossus, the sense organs are poorly developed. In the epidermis are numerous neurosensory cells which are connected to the nerve net, they are more numerous on the proboscis.
It is claimed that in some species a few neurosensory cells form photoreceptors sensory to light. On the ventral side of the base of the proboscis is a U-shaped depression called a preoral ciliary organ. It has ciliated cells joined to the nerve net, it is a chemoreceptor.
11. Reproductive System of Balanoglossus:
In Balanoglossus, the sexes are separate and are indistinguishable externally except in case of the colour of the ripe gonads shown through the body wall in the living animal.
The gonads occur in one or more longitudinal rows to the sides of the alimentary canal lying within the genital pleurae in the anterior part of the trunk. Gonads develop from the coelomic wall, though they have no connection with coelom in the adult.
The gonads are generally sacciform bodies but may be elongated or lobulated and secondary gonads may arise by subdivision of the primary ones through lobulation. Each gonad is a sac, it continues into a short ductule which opens to the exterior by a gonopore. The gonopores are generally located to the lateral (external) side of the gill-pores in the same branchio-genital groove.
The saccular gonads are lined with germinal epithelium which is continuous with the ectoderm. By the proliferation of cells from the germinal epithelium sperms or ova are produced. The mature sperms and ova are discharged outside through the genital pores. The sperm has a rounded head and a flagella-like tail but the ova are of two types.
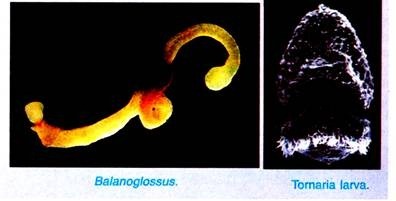
The small ovum measures about 0.06 mm in diameter and undergoes indirect development with a pelagic larva known as tornaria larva, while the larger ovum measures about 0.4 mm in diameter and undergoes direct development without larval stage. The mature sperms and ova are shed into the burrows where fertilisation takes place, i.e., the fertilisation is external.
Asexual Reproduction:
Asexual reproduction is known to occur in Balanoglossus capensis (Gilchrist, 1923). During summer the juvenile phase of this, at first considered a distinct species for it lacks hepatic sacculations, reproduces by cutting off small pieces from the tail end forward. These regenerate completely into the adult sexual type found in winter.
Regeneration:
Balanoglossus has great power of regeneration, small pieces are constricted from the posterior end, each of which regenerates into a complete individual. Other broken pieces of the animal also regenerate into new individuals.
A detailed study of regeneration was made by Dawydoff (1902, 1907, and 1909) for Gloss, minutus and by Rao (1955) for Ptychodera flava. The isolated proboscis, with or without the collar, lines and moves about for some time but appears incapable of regenerating posterior structures. Pieces of trunk regenerate completely in both species.
12 Development of Balanoglossus:
(i) Fertilisation in Balanoglossus:
During breeding season (May to June) mature ova and sperms are discharged in the surrounding water where fertilisation takes place. First the ova, egg-mass, are discharged by the female from its burrow and then the sperms are discharged by the male from its burrow. The number of eggs discharged at a time varies from few dozens to more than a thousand. Normally one to three hundred eggs are shed at a time.
According to available evidences maturation starts some four hours before ovulation and that the egg is generally in the metaphase of the first meiotic division when shed. It is at this condition the egg is fertilizable.
Fertilisation of eggs within 6 to 7 hours after shedding yields a high percentage of normal development. The spermatozoon is able to enter the eggs at any point over the entire surface. After fertilisation, the cleavage starts.
(ii) Pre-Larval Development in Balanoglossus:
The zygote, produced as a result of fertilisation, undergoes cleavage. The cleavage is holoblastic, almost equal and mostly of the radial type. The first cleavage starts about two hours after fertilisation and produces two generally, but not invariably, equal cells. The second cleagave is like the first and produces usually (but not invariably) four approximately equal cells.
As a result of third and subsequent cleagaves a sphere of equal blastomeres is produced, it is called morula. The morula undergoes the re-organisation of its blastomeres and takes the form of a single-layered hollow and spherical blastula or coeloblastula. Its central fluid-filled cavity is called the blastocoel. As the cells multiply the volume of blastula increases. Blastula results in about 6-15 hours after fertilisation.
Within 12-24 hours, an invagination starts in the blastula which deepens to form the archenteron. The archenteron opens to the outside through a blastopore. The blastopore marks the posterior end of the embryo. The blastopore soon closes and the embryo now called gastrula.
The gastrula elongates along the anteroposterior axis. Now the anterior tip of the archenteron is differentiated as a coelomic vesicle called the protocoel. Thus, origin of coelom is enterocoelic. The remaining posterior part of the archenteron marks the future gut or alimentary canal.
The protocoel becomes triangular in shape. One end of the protocoel gets attached to the underside of the apical thickening and another end opens to outside through an aperture, the hydropore, towards the dorsal side of the embryo.
The protocoel and hydropore represent the future proboscis coelom and proboscis pore respectively. The collar and trunk coelom develop as solid invaginations of the hindgut, independent of the formation of protocoel.
(iii) Larval Development in Balanoglossus:
After the formation of the protocoel, the inner end of the early gut moves towards the ventral surface and opens to the outside through a mouth. The gut is now differentiated into the oseophagus, stomach and intestine. The intestine opens to outside through an anus, formed at the place of closed blastopore.
By this time (after a day or so) the embryo becomes uniformly ciliated and escapes from fertilisation membrane to lead a free swimming larval life. It is called tornaria larva.
(iv) Tornaria Larva in Balanoglossus:
Tornaria larva was first of all discovered by J. Muller in 1850 and was considered by him as the larva of echinoderms. Later on in 1869 it was Metschnikoff who established that it is a larva of Balanoglossus clavigerus. The name tornaria is given to it because of its habit of rotating in circles. Tornaria larva is usually oval in shape and is excessively transparent.
The size of tornaria larva varies from 1 mm to 3 mm. It has a ventral mouth near the equatorial plane of the body, a posterior terminal anus and gut differentiated into an oesophagus, stomach and intestine. The cilia form two bands on the body surface. The anterior ciliary band or circumoral band takes up a winding course over the preoral surface and forms a postoral loop. Its cilia are short and serve to collect the food.
The posterior ciliated band or circumanal ring or telotroch lies as a ring in front of the anus. The cilia in this band are long, powerful and act as chief locomotor organ of tornaria. A ciliary wave passing along the telotroch causes the larva to rotate constantly in swimming. At the anterior end is an apical plate of thickened epidermal cells.
The apical plate bears a pair of eye spots or ocelli and a tuft of sensory cilia called apical tuft or ciliary organ. The protocoel (proboscis coelom) is present in the form of a thin-walled sac and opens to the exterior through a hydropore (proboscis pore). To the right of the hydropore lies a pulsating heart vesicle which develops in the later stages of tornaria larva. The collar and trunk coeloms appear in the older larva.
(v) Metamorphosis in Balanoglossus:
The tornaria larva swims freely, leads a planktonic life feeding on minute organisms. After swimming for some time the tornaria larva sinks to the bottom and metamorphoses into an adult. During metamorphosis, the size of larva is reduced probably due to loss of water. Transparency, ciliary bands, sensory cilia and eye spots are lost.
The body elongates and is distinguished into proboscis, collar and trunk by the appearance of two constrictions, and the trunk region is elongated.
The hydropore persists as proboscis pore. Simultaneously the buccal diverticulum and gill-slits appear as outgrowth of the alimentary canal. The reproductive organs make their appearance, probably from the mesoderm. Thus, the larva gradually changes into the adult. The adult leads a benthonic life.
13. Affinities of Balanoglossus:
The position of Hemichordata, in the scheme of classification of animals, has been controversial. In 1814, Sedgwick and Huxley suggested the affinities of Enteropneusta (Hemichordata) with the vertebrates and it was in 1885 Bateson considered this group as a subphylum of the phylum Chordata.
Metschnikoff (1865) stated that Enteropneusta had certain affinities with Echinodermata. Spengel (1893) showed the relationship of Enteropneusta with Annelida.
But on the basis of general organisation, some recent workers, such as Van der Horst (1939), Dawydoff (1948), Marcus (1958) and Hyman (1959) have thought it proper to remove this group from phylum Chordata to give it the status of an independent invertebrate phylum.
The name “Hemichordata” is, however, retained for the group because it suggests that its members are related to chordates, i.e., they are “half’ or “part” chordates, a fact that is undisputed.
Affinities of Balanoglossus (Enteropneusta, Hemichordata) with chordates and non-chordate phyla are as follows:
Affinities with Chordata:
Bateson (1887) included Hemichordata in phylum Chordata, since then a close relationship has been acknowledged between hemichordates and chordates. This arrangement exists even today in most books.
Resemblances:
The phylogenetic relationship of hemichordates and chordates is based on the supposed presence of the three fundamental chordate characters in both groups, viz., a notochord, central nervous system and gill-clefts.
The buccal diverticulum or stomochord of hemichordates has been regarded as the equivalent of a notochord since the time of Bateson.
Modern workers of hemichordates do not accept this idea and have raised many objections:
1. The buccal diverticulum is a hollow evagination of the anterior wall of the buccal cavity, and it is not definite whether it is endodermal or ectodermal in origin, whereas the notochord is a long solid rod formed from the roof of the archenteron.
2. The buccal diverticulum is generally made of ordinary epithelial cells, while the notochord of vertebrates consists of large vacuolated cells.
3. The buccal diverticulum has no enclosing sheath as found around the notochord.
4. The buccal diverticulum lies ventral to the dorsal blood vessel, whereas the vertebrate notochord is always dorsal to the main dorsal blood vessel.
5. The buccal diverticulum is small and confined to the proboscis, while the notochord extends far backwards. It can be safely concluded that there is no representative of the notochord in hemichordates.
There are certain resemblances between the nervous system of hemichordates and chordates, such as its position, formation of the dorsal nerve cord from the dorsal epidermis, and the hollow collar cord which often has a neuropore and is comparable with the neural cord of vertebrates.
But there are major differences, such as its superficial position in contact with the epidermis, possession of a main ventral nerve cord, and a circumenteric nerve ring, in these features the nervous system is distinctly invertebrate. Thus, the invertebrate features of the nervous system of hemichordates outweigh its chordate characters.
The chief link between the hemichordates and chordates lies in the pharynx and its gill-clefts.
The details of the branchial apparatus having tongue bars, M-shaped skeletal rods and synapticula are exactly like those of Amphioxus. But the endostyle and epibranchial groove are absent from the pharynx of hemichordates. Such similarity can be only due to common ancestry, and phylogenetic relationship of hemichordates and chordates cannot be denied.
Differences:
But the inclusion of hemichordates in phylum Chordata cannot be justified on the basis of a few similarities which are more than outweighed by important differences.
The main differences are:
1. Chordates do not have the body and coelomic regions corresponding to those of hemichordates.
2. The circulatory and nervous systems of hemichordates are like those of invertebrates.
3. There is no post-anal tail in hemichordates.
4. Chordates are metamerically segmented animals, this segmentation is clearly shown by the muscular, nervous, circulatory, and excretory systems, whereas there is a total absence of segmentation in hemichordates.
Affinities with Annelida:
Resemblances:
The main resemblances of Hemichordata with Annelida are as follows:
1. The general body form and burrowing habit of tubicolous forms are alike and mud is ingested in burrowing. It is passed out from the anus as castings.
2. The vascular system of most hemichordates is like that of annelids with blood flowing anteriorly in the dorsal vessel and posteriorly in the ventral vessels.
3. The hemichordate tornaria larva appears like a modified trochosphere larva of polychaete worms.
Differences:
The differences between two groups are so great that there can be no phylogenetic relationship between them.
The differences are as follows:
1. Gill-slits are absent in annelids.
2. Paired nerve cords are present in annelids.
3. The larva of Hemichordata and Annelida also differ in the following ways:
(i) Nephridia are absent in tornaria larva.
(ii) Pre-oral coelom is absent in trochosphere larva.
(iii) In trochosphere blastopore becomes the mouth, while in tornaria it becomes the anus.
Affinities with Echinodermata:
The adult hemichordate and adult echinoderm are so different that one cannot suspect any relationship between them, the only anatomical similarity between them is their nervous system which in both cases consists of nerve net lying near the surface embedded in the epidermis.
But there is a strong affinity between the two phyla on embryological evidence, the method of formation of the gastrula and the coelom is very similar in the two phyla and for years the tornaria larva was considered to be the larva of an echinoderm. The tornaria larva shows a very striking resemblance with the auricularia larva and specially with bipinnaria of Asteroidea.
Resemblances:
The resemblance extends into the following details:
1. The ciliated band is identical and follows the same course in the tornaria and the auricularia and bipinnaria, though the telotroch and eye spots of the tornaria are absent in echinoderm larvae.
2. The alimentary canal has the same shape and the same divisions into foregut, stomach, and intestine in hemichordate and echinoderm larvae.
3. In both groups the blastopore becomes larval anus.
4. The cleavage and gastrulation follow the same pattern in both.
5. The greatest and the most convincing resemblance lies in the method of formation and arrangement of coelomic cavities.
In both the coelom is of enterocoelous origin and it divides into three antero-posterior parts, which in hemichordates are called proboscis coelom (protocoel), collar coelom (mesocoel), and trunk coelom (metacoel), while in echinoderms the three parts of the coelom are axocoel, hydrocoel, and somatocoel.
Moreover the proboscis coelom and collar coelom of hemichordates open to the exterior by pores through short hydroporic canals, as does the hydrocoel in echinoderms.
6. The heart vesicle of hemichordates is related to the proboscis coelom and is homologous with the madreporic vesicle of echinoderrm larvae, and both these structures are closely connected to the glomerulus of hemichordates and the axial gland of echinoderms which combine the vascular and excretory functions.
Differences:
There are following differences between two groups:
1. Eye spot is absent in bipinnaria.
2. The apical plate and telotroch are absent in bipinnaria.
3. The protocoel is paired in echinoderms, while unpaired in tornaria larva.
The many embryonic resemblances between hemichordates and echinoderms cannot possibly be accidental or due to convergent evolution. The only infallible conclusion is that the two groups are closely related and that they arose from a common ancestor.
Echinoderms have deviated greatly from the ancestral type, while hemichordates are closer to it. The common ancestor gave rise to echinoderms as a blind side branch, while the main line of evolution produced the hemichordates and chordates.
Conclusion:
The above affinities have led to the conclusion that echinoderms, hemichordates and chordates have arisen from a common ancestral stock, the dipleura larva. Further, the echinoderms deviated greatly from the ancestral stock and formed blind branch in the main line of evolution. The main line of evolution continued to give rise to hemichordates and chordates.
It appears most reasonable to place them in the invertebrates as an independent phylum which has arisen from an ancestral stock that has given rise, on the one hand, to echinoderms and, on the other hand, to hemichordates and chordates.