In this article we will discuss about the metabolism of iron in human body with the help of suitable diagram.
Distribution of Iron:
Iron is distributed in the body:
(a) As iron porphyrins in haemoglobin, myoglobin, and also
(b) As iron enzymes in catalase, cytochrome and peroxidases.
Besides these, the iron is also present as non-iron porphyrins in transferrin, ferritin and haemosiderin.
All animal food, e.g., meat, liver, egg, etc., excepting milk and butter, vegetables, e.g., peas, lentils, green leaves, fruits.
Daily Requirement of Iron:
At least 12 mgm, better 15-20 mgm, per day. Generally enough in the normal diet. Pregnant and lactating females should have more. Milk being deficient in iron, the infant may develop anaemia. The foetal liver contains a large store of iron which is used up in the first three months. After third month, infants should have added amounts.
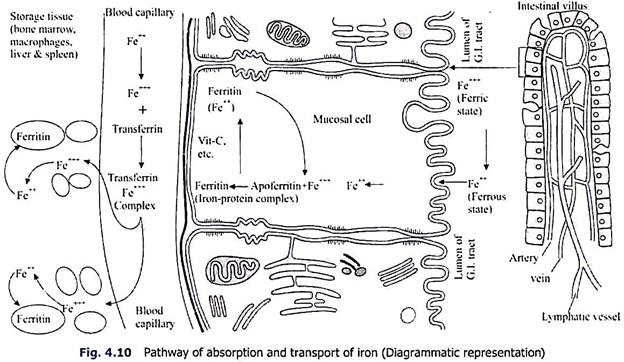
Absorption and Transport of Iron:
Iron is absorbed mostly from the whole of the gastro-intestinal tract but a large amount is absorbed from the upper part of the small intestine particularly the duodenum. Dietary iron is absorbed through the mucosal cells as ferrous (Fe++) form. Iron in diet is mostly present as ferric (Fe+++) state which is reduced to ferrous form during’ absorption. Vitamin C, glutathione and amino acid-SH groups help in reduction of ferric to ferrous form.
After entering the mucosal cell as ferrous form, the iron molecules are rapidly reconverted into ferric state. The ferric iron as ferric-hydroxide phosphate combines with a protein, apoferritin of the mucosal cells with the formation of iron-phosphorus protein complex, ferritin. This ferritin is one of the storage forms of iron in the tissue.
It is claimed that homeostasis of iron content in the body is maintained not through excretion but through controlling the absorption in the G. I. tract. It has been postulated by Granick (1954) that at the border of the mucosal cells, the saturated apoferritin acts as a mucosa block and further absorption of iron is prohibited. But at present it is believed that apoferritin mechanism does not tend to stabilise the iron absorption.
From the mucosal cell the ferritin iron passes into the blood. At first the ferritin iron is reduced into ferrous form and as such enters the blood stream. Here vitamin C also helps in transformation of ferric to ferrous form. However, the ferrous iron after entering the blood stream is re-oxidised into ferric form and combines with β1-iron-binding globulin, transferrin or siderophilin of the plasma.
It has been described by Osaki and others (1966) that ceruloplasmin (copper-binding protein) of the plasma also exerts a catalytic activity in plasma to convert Fe++ to Fe+++ form and thus incorporation of iron in the plasma transferrin is hastened. Transferrin iron complex is the transport form of iron of the plasma and is carried to the myeloid tissue, liver, spleen, lymph node and other tissues of the body.
Transferrin is thus the iron binding protein of the plasma and it shuttles iron atoms between tissues without itself being utilised appreciably. The transferrin can bind two atoms of Fe+++ per molecule of protein to form the red-coloured ferric protein complex.
Normal protein-bound iron (PBI) in the plasma of males is approximately 120-140 µg percent, and that of in females is 90-120 µg per cent. The total iron-binding capacity (TIBC) is about 300 to 360 µg per 100 ml in both sexes.
In the capillary blood vessels, the Fe++++ of transferrin passes through the peripheral capillary wall directly into the tissue spaces. But from the tissue spaces, the iron enters the tissues as ferrous (Fe++) state and is stored as ferritin (Fe+++) state (Fig. 4.10). If the parenteral administration of iron exceeds the capability of the tissue to store as ferritin, then the excess is stored as haemosiderin.
Absorption of iron depends upon the following factors:
i. Iron Requirement of the Subject:
In some unknown way, the degree of immediate need of the body for iron determines the rate and amount of absorption from the small intestine. Miller (1954) suggests that iron absorption is under the control of a regulatory mechanism geared to the erythron need rather than to the reserve stores.
Other workers suggest that reserve iron stores control iron absorption via the mucosal cell through a low oxygen supply induced by low Hb in the blood stream. The ferritin –Fe+++ stored in the mucosal cell, its shifting towards Fe++ for diffusion into blood and subsequent conversion to ferritin –Fe+++ prevent loss of iron into the intestinal tract. Ferritin with 17 to 23% of Fe is a ferric compound of the colourles apoferritin (protein) and colloidal ferric hydroxide phosphate by weight.
Iron absorption is increased during growth, menstruation, pregnancy and in blood disorders. Absorption is increased markedly when the iron needs are acute as in haemorrhage or in anaemias.
ii. Form of the Compound:
It is said that iron is best absorbed in ferrous (Fe++) form. Most of the iron taken with food is in ferric (Fe+++) form. They are at least partly converted into ferrous compounds before absorption. Organic iron of food is much less available for absorption than the inorganic. Insoluble forms are not absorbed.
iii. Reaction of the Gastro-Intestinal Contents:
The acidity of the gastric juice helps absorption. The gastric HCl helps the liberation of iron from the organic compounds in diet. Reduction from ferric form to ferrous one takes place in stomach with the help of gastric secretions. Partial gastrectomy often leads to iron- deficiency anaemia.
iv. Pigments:
Absorption of iron is increased by chlorophyll and bile pigments. It is believed by certain workers that bile or gastric HCl is not needed for the absorption of inorganic iron salts.
v. Calcium and Vitamin C:
A small amount of Ca decreases the formation of insoluble iron phosphates and thus helps absorption, but large amounts of Ca inhibit iron assimilation. Vitamin C increases the absorption of iron from foods, possibly by reducing the ferric iron to the ferrous state.
Absorption is retarded by excessive mucus, administration of alkalies or low gastric acidity.
Time Taken for Absorption:
The rate of absorption of iron is determined by the iron requirement for Hb synthesis. In anaemic cases, after a single dose of iron, a rise of serum iron takes place in 30 minutes, reaching its maximum in 3-5 hours (0.35 mgm%, compared to normal value, 0.10 mgm %) and returns to normal in about 12 hours. Maximum absorption is completed in 18 hours. [In normal animals little iron needs to be absorbed, because very little is excreted. The body observes a rigorous principle of economy in this respect.]
Iron derived from the disintegration of the red cells remains stored, and is utilised for the synthesis of further haemoglobin. If a man be in iron balance -any excess of iron administered per mouth will not be absorbed, but passes out in the faeces. If iron salts are given by injections, only traces appear in the urine, the rest remains stored in the reticulo-endothelial cells as haemosiderin. Haemoglobin in the blood falls when iron loss exceeds, that of iron absorption and anaemia develops.
Iron in Blood:
Whole blood contains about 45-50 mgm of iron per 100 ml. The total quantity present in all the red cells is about 3 gm. Another 1-3 gm is present in the rest of the body.
Iron is present in blood in two forms:
i. Plasma Iron:
Only traces of iron (average 0.10 mgm per 100 ml) is present in plasma. It represents the form in which iron is transported in blood, from place to place, the compound known as transferrin or siderophilin. It is increased when the red cell formation is diminished, e.g., in Aplastic anaemia, Pernicious anaemia, etc. It is diminished when there is rapid red cell formation.
ii. As Haemoglobin:
This accounts for about 92-98% of the total blood iron. It corresponds to about 50mgm of inorganic iron per 100 ml of blood.
Storage of Iron:
Iron is stored in two forms; ferritin and haemosiderin. The former is water-soluble while the latter is granular and insoluble in water. Reticulo- endothelial system in general, particularly, liver, spleen and bone marrow store iron.
Liver iron is readily increased by iron administration. In condition of excess haemolysis iron is deposited in these places in large quantities. Normally the iron liberated from the breakdown of red blood cells is also stored in these places and is utilised for Hb synthesis.
Excretion of Iron:
Only in traces in urine, bile and faeces. In an adult the urinary loss is on the average 0.2 mgm per day. It is believed that the iron content of the body is controlled by regulation of its absorption and not by excretion. Iron loss occurs during pregnancy, during labour, in the menstrual period, due to loss of blood, etc. (Fig. 4.11).
Functions of Iron:
i. Formation of Hb:
The primary function of iron is to form haemoglobin.
ii. Development of Red Cells:
Iron is not only necessary for Hb synthesis but also for the formation and maturation of red cells.
iii. Oxygen Carriage in Blood:
Oxygen carriage in blood in the form of Hb. One gm of Hb carries about 1.34 ml of oxygen.
iv. Related to Tissue Oxidation:
a. Cytochrome is an iron-containing compound. It is concerned with the oxidation of metabolites in the cells.
b. Indophenol oxidase is also an iron compound.
v. Supplies O2 to the Muscle:
Myoglobin of muscle is an iron-containing chromoprotein like haemoglobin. It combines with O2 and acts as an oxygen store for muscle.
vi. Relation with the Cell Nucleus:
The chromatin of the nucleus contains iron. It is possible that this iron takes an essential part (may be oxidative) in the functions of nuclei.
vii. Relation with Oxidation in Nerve Cells:
Nissl granules, present in the cytoplasm of the nerve cells, contain organically combined iron. Here, iron serves some essential roles probably in oxidation. These granules disappear during activity of the nerve cells, and reappear during rest.
Deficiency of Iron:
Iron deficiency causes secondary anaemia (Microcytic, Hypochromic). The haemoglobin content of the red cells is diminished. The size and volume of the red cells are below average. There is normoblastic hyperplasia in the red bone marrow. Iron-deficiency anaemia occurs in children and adults due to severe blood loss.