In this article we will discuss about the production of various bio-fertilizers.
Contents
1. Production of Bacterial Bio-Fertilizer:
With day-by-day increasing the population, especially in developing countries like India, the stress on agriculture is also increasing continuously. With the development, the land area under farming is not increasing but is further decreasing, this has posed extra burden on the agriculture. Therefore, the land available for agriculture should be economically utilized and maximum results be obtained.
Most of our agricultural lands are deprived of either one mineral or the other. These minerals are essential for the growth and development of plants. One of the nutrients for any type of plant is nitrogen. Nitrogen is a major element required by the plant for growth and development. The nitrogen is provided in the form of chemical fertilizer.
Such chemical fertilizers pose health hazards and pollution problem in soil besides these are quite expensive, bringing the cost of production much higher.
Therefore, bio-fertilizers are being recommended in place of chemical fertilizers. Bio-fertilizers are the formulations of living microorganisms which are able to fix atmospheric nitrogen in the available form for plants (nitrate form) either by living freely in the soil or associated symbiotically with plants.
Although nitrogen fixers are present in the soil, enrichment of soil with effective microbial strains is much beneficial for the crop yields. Use of composite bio-fertilizers can increase soil fertility.
It has been proved that bio-fertilizers are cost effective, cheap and renewable source to supplement the chemical fertilizers:
(i) History:
In 1895, Nobbe and Hiltner applied for patents in England and the United States for a legume inoculant that was later marketed as Nitragin. Nitragin was produced on gelatin and agar nutrient media.
However, agar based inoculants were soon replaced by peat-based ones because in agar based inoculants, mortality was very high during the dry phase. In India, the production of bio-fertilizers on commercial scale was started only during late 1960’s when yellow seeded soybean was introduced for the first time.
Recognition of Indian peat as suitable carrier for production of bio-fertilizer in 1969 further augmented, the growth of bio-fertilizer industry in India.
The performance of Indian peat-based bio-fertilizer at Indian Agricultural Research Institute, New Delhi, was found to be comparable to that obtained with imported ‘Nitragin’ bio-fertilizer from the U.S.A. Since then, the process of development of bio-fertilizer, specially of rhizobial bio-fertilizer for various crops in India has made a tremendous success.
(ii) Production of Bio-Fertilizer:
In order to meet the food requirements of ever increasing population, the nitrogen fertilizer requirement for crop production by 2000 A.D. was estimated to be about 11.4 × 106 tonnes. Biological nitrogen fixation can be the key to fill up this gap because of high cost and several other demerits of chemical fertilizers.
For production of a good and efficient bio-fertilizer, first of all an efficient nitrogen fixing strain is required, then its inoculum (the form in which the strain is to be applied in fields) is produced.
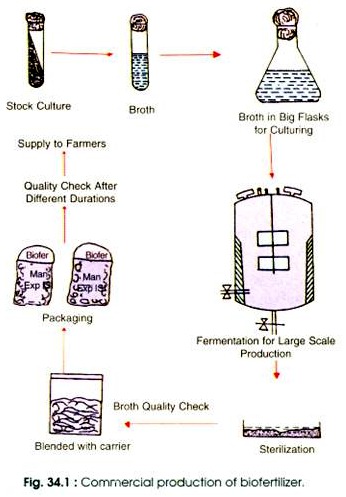
Packing, storing and maintenance are other aspects of bio-fertilizer production. While producing bio-fertilizers the standards laid down by BIS have also to be kept in mind for making the product authentic. Commercial production of bacteria, involved in the production of bio-fertilizer is shown in Fig. 34.1.
(iii) Criteria for Strain Selection:
The efficient nitrogen fixing strain is evolved or selected in laboratory, maintained and multiplied on nutritionally rich artificial medium before inoculating the seed or soil. In soil, the strain has to survive and multiply to compete for infection site on roots against hostile environment in soil.
(iv) Steps for Preparing Bio-Fertilizer:
The isolated strain is inoculated in small flasks containing suitable medium for inoculum production. The volume of the starter culture should be a minimum of 1% to obtain atleast 1×109 cells/ml. Now the culture obtained is added to the carrier for inoculant (bio-fertilizer) preparation.
Carriers carry the nitrogen fixing organisms to the fields. In some cases carrier is first sterilised and then inoculated, while in other cases it is first inoculated and then sterilised by UV irradiation. The inoculum is now packed with 109-1010 viable cells per gram. Final moisture content should be around 40-60%. For large scale production of inoculum, culture fermenters are used.
(a) Seed Pelleting:
Direct seed coating with the gum arable or sugary syrup and useful nitrogen fixing strains especially the coating of rhizobia over specific host legume seeds are another method for obtaining fruitful results. As before, first of all the inoculum is prepared of the desired strain and then the seeds are inoculated by using either direct coating method or slurry method. Immediately after seed coating, CaCO3 is added to sticky seeds.
The practice of seed inoculation dates back to 1896 when Voecher used this technique. In many soils the nodule bacteria are absent or are not adequate in either number or quality to meet the nitrogen requirements of the plants. Under these conditions, it is necessary to inoculate seeds or seedlings with highly effective rhizobia.
(b) Inoculant Carriers:
Most inoculants are the mixture of the broth culture and a finely milled, neutralized carrier material. Carrier is a substance having properties such as, non-toxicity, good moisture absorption capacity, free of lump forming material, easy to sterilize, inexpensive, easily available and good buffering capacity, so that it can prolong and maintain the growth of nitrogen fixing microorganisms which it is carrying.
The most frequently used carrier for inoculant production is peat. However, peat is not available in certain countries such as India. A wide range of substitutes e.g. lignite, coal, charcoal, bagasse, filter mud, vermiculite, polyacrylamide, mineral soils, vegetable oils, etc. have been tested as alternative carriers.
Carrier processing e.g. mining, drying and milling are the most capital intensive aspects of inoculant (bio-fertiliser) production. First of all the carrier like peat is mined, drained and cleared off stones, roots, etc. Then, it is shredded and dried.
The peat is then passed through heavy mills. Material with a particle size of 10-40 µm is collected for seed coating. Peat with particle size of 500-1500 µm is used for soil implant inoculant. Carriers have to be neutralised by adding precipitated calcium carbonate (pH 6.5-7.0). After this, the carriers are sterilized for use as inoculants.
(c) Quality Standards for Inoculants:
Like every product, the bio-fertilizers should also follow certain standards. The inoculant should be carrier-based and should contain a minimum of 108 viable cells per gram of carrier on dry mass basis within 15 days of manufacture, and 107 within 15 days before the expiry date marked on the packet when the inoculant is stored at 25-30°C. The inoculant should have a maximum expiry period of 12 months from date of manufacture. The inoculant should not have any contaminant.
The contamination is one of the biggest problems faced by the bio-fertilizer industry. The pH of inoculant should be between 6.0 and 7.5. Each packet containing the bio-fertiliser should be marked with the information’s e.g, name of product, leguminous crop for which intended, name and address of manufacture, type of carrier, batch or code number, date of manufacture, date of expiry, net quantity meant for net area and storage instructions. Each packet should also be marked with ISI (BIS) certification mark.
The inoculant (bio-fertilizer) should be stored in a cool place away from direct heat preferably at a temperature of 15°C. The bio inoculant should be packed in 50-75 µ low density polyethylene packets.
Two main methods of inoculation are currently being used (a) seed inoculation and (b) soil inoculation. The soil inoculation is done by delivering the inoculant directly into the sowing furrow with the seeds. Seed inoculation by pelleting or coating the seed with inoculant is the most popular methods.
(v) Green Manuring:
Green manuring is defined as a “farming practice where a leguminous plant which has derived enough benefits from its association with appropriate species of Rhizobium is ploughed into the field soil and then a non -legume is sown and allowed to get benefitted from the already present nitrogen fixer”.
The practice of green manuring began from time immemorial from several century B.C. in India and China. During the course of time, availability of chemical fertilizers decreased the significance of green manuring. In recent years, due to hike in price of chemical fertilizers, the practice of green manuring is reemphasized.
Some of the cultivated legumes and annual legumes such as Crotolaria juncea, C. striata, Cassia mimosoides, Cyamopsis pamas, Glycine wightii, Indigofera linifolia, Sesbania rostrata, Leucaena leucocephala, etc. contribute nitrogen.
In addition to nitrogen, green manuring provides organic matter, phosphorus, potassium besides minimising the pathogenic organisms in soil. The reclamation of “usar lands” can also be done by green manuring.
In India besides a large number of private and semi-Government organisations, the National Bio-fertilizer Development Centre sponsored by the Ministry of Agriculture and the establishment of National Centres for blue green algal collections at IARI, New Delhi, the Department of Biotechnology, Govt. of India, Ministry of Science & Technology are the major developments that reflect our concern to harness bio-fertilizers in our agricultural economy.
2. Algal and Other Bio-Fertilizers:
Biological nitrogen fertilizers play a vital role to solve the problems of soil fertility, soil productivity and environmental quality. Anabaena azollae, a cyanobacterium lives in symbiotic association with the free floating water fern Azolla.
The symbiotic system Azolla-Anabaena complex is known to contribute 40-60 kg N ha-1 per rice crop. Anabaena azolle can grow photo autotrophically and fixes atmospheric nitrogen. The nitrogen fixing cyanobacteria such as A. azollae and variabilis when immobilized in polyurethane foam and sugar cane waste have significantly increased the nitrogen fixing activity and ammonia secretion.
The inoculation of cyanobacteria in nee crop significantly influenced the growth of rice crop by secretion of ammonia in flood water. The use of neem cake coupled with the inculation of Azolla greatly increased the nitrogen utilization efficiency in rice crop.
Besides Anabaena, other nitrogen fixing cyanobacteria like Aulosira, Calothrix, Hapalosiphon, Scytonema, Tolypothrix and Westiellopsis have been held responsible for the spontaneous fertility of the tropic rice fields.
In addition to contributing N, the cyanobacteria add organic matter, secrete growth promoting substance like auxins and vitamins, mobilise insoluble phosphate and improve physical and chemical nature of the soil. Algalization has been shown to ameliorate the saline- alkali soils, help in the formation of soil aggregates, reduce soil compaction, and narrow C: N ratio.
These organisms enable the crop to utilize more of the applied nutrients leading to increased fertilizer utilising efficiency of crop plant. Most of the cyanobacteria act as supplements to fertilizer N contributing up to 30 kg N ha-1 season-1. The increase in the crop yield varies between 5-25 percent.
(i) Mass Production of Cyanobacterial Biofertilizers:
For outdoor cultivation of cyanobacterial biofertilizers, the regional specific strain should be used. In such practices, a mixture of 5 or 6 regionally acclimatized strains of cyanobacteria e.g. species of Anabaena, Aulosira, Cylindrospermum, Gloeotrichia, Nostoc, Plectonema, Tolypothrix etc. are generally used as starter inoculum.
The following methods are used for mass cultivation:
(a) Cemented tank method,
(b) Shallow metal troughs method,
(c) Polythene lined pit method and
(d) Field method.
The polythene lined method is most suitable for small and marginal farmers for the preparation of bio-fertilizer. In this method, small pits are prepared in field and lined with thick polythene sheets.
The mass cultivation of cyanobacteria is done by using any of the above four methods; the steps are given below:
(a) Prepare the cemented tank, shallow trays of iron sheets or polythene lined pits in an open area. Width of tanks or pits should not be more than 1.5 m. This will facilitate the proper handling of culture.
(b) Transfer 2-3 kg soil and add 100 g superphosphate. Water the pit to about 10 cm height. Mix lime to adjust the pH. Add 2 ml of insecticide to protect the culture from mosquitoes. Mix well and allow to settle down soil particles.
(c) When water becomes clear, sprinkle 100 g starter culture on the surface of water.
(d) When temperature remains around 35-40°C during summer, optimum growth of cyanobacteria is achieved. The water level is always maintained about 10 cm during the period.
(e) After drying, the algal mass (mat) is separated from the soil that forms flakes. During summer about 1 kg pure algal mat per m2 area is produced. It is collected, powdered, and packed in polythene bag and supplied to the farmers after sealing the packets.
(f) The algal flakes can be used as starter inoculum again.
(ii) Mass Cultivation of Azolla:
The aquatic heterosporus fern contains endophytic cyanobacterium, Anabaena azollae in its leaf cavity. There are number of species of Azolla, namely A. caroliniana, A, filiculoides, A. maxicana, A. nilotica, A. pinnata and A. rubra which are used as biofertilizer especially for paddy. For mass cultivation of Azolla, microplots (20 m2) are prepared in nurseries in which sufficient water (5-10 cm) is added.
For profuse growth of Azolla, 4-20 kg P2O5/ha is also amended. Optimum pH (8.0) and temperature (14-30°C) should be maintained. Finally, microplots are inoculated with fresh Azolla (0.5 to 0.4 kg/m2). An insecticide (Furadon) is used to check the insect’s attack. After 3 weeks, the mat of Azolla is ready for harvest and the same microplot is inoculated with fresh Azolla to repeat the cultivation.
Azolla mat is harvested and dried to use as green manure.
There are two methods for its application in field:
(a) Incorporation of Azolla in soil prior to rice cultivation, and
(b) Transplantation of rice followed by water draining and incorporation of Azolla.
However, reports from the IRRI, Manila (Philippines) revealed that growing of Azolla in rice field before rice transplantation increased the yield equivalent to that obtained from 30 kg/ha nitrogen as urea or ammonium phosphate.
3. Endophytic Nitrogen Fixers:
Recently, several non-leguminous and particularly graminaceous species such as rice, wheat and forage grasses have registered tremendous interest in nitrogen fixation. Isolation of a number of diazotrophic bacteria such as Azospirillum, Herbaspirillum and Acetobacter is reported.
The term endophyte refers to microorganisms (bacteria and fungi) that colonize root interior of plants and live most of their life inside the plant tissue. Splitting the term endophyte into facultative and obligate was suggested to distinguish, respectively, strains that are able to colonize both the surface and root interior and to survive well in soil from those that do not survive well in soil but colonize the root interior and aerial parts.
(i) Facultative Endophytic Diazotrophs:
This group is composed of Azospirillum spp. and considered important with non-legume plants. Although A. lipoferum was the first species of the genus isolated by Tarrand (1978). A. brasilense among all the seven known species is the best characterized at physiological and molecular levels.
(ii) Obligate Endophytic Diazotrophs:
This group includes Acetobacter diazotrophicus (syn. Gluconacetobacter diazotrophicus) a nitrogen fixing bacterium clustered in the alpha sub-class of the proteobacteria, Azoarcus spp., Herbaspirillum spp. and a partially identified Burkholderia sp. are clustered in the beta sub-class of the proteobacteria.
(iii) Other Bacteria:
Alcaligens, a diazotrophic member of this genus has been consistently isolated from the rhizosphere of wet rice land. Burkholderia, the other bacterium appears to have potential as rice inoculant. In the case of Klebsiella, substituted nitrogen fixation has been observed in rice inoculated with K. oxytoca or any other Klebsiella spp. that are considered as endophytes.
The diazotrophic nature of some members of the genus Pseudomonas is still a matter of debate. Nevertheless, several bacteria within it are clearly diazotrophic such as Pseudomonas diazotrophicus, P. flurorescens, P. saccharophila and P. stutzeri. Recently, several researchers have attempted to construct an artificial association between rhizobia and rice particularly with Azorhizobium caulinodans.
(a) Isolation and Identification of Endophytes:
For isolation and identification of natural diazotrophs from plant samples, root or stem, washed with sterile water, surface sterilized with 70% ethanol for 5 minutes and with sodium hypochlorite (2-5%) for 30 second, washed several times using sterile water. Sterilization of root and stem will be verified by rolling them on BMS agar plates.
Then homogenize the sample in a mortar and pestle in sterile phosphate buffer, saline 1% sugar solution and serially diluted and 0.1 ml sample transfer into vials containing 5-8 ml of respective semisolid media for the targeted bacterium with respective C sources with an initial pH of 6.0.
The number of diazotrophic populations is determined by the most probable number methods using a McCrady table. Vials with veil pellicles reaching the surface after incubation at 30°C with or without gas production and with positive reaction for acetylene reduction activity, show the presence of good endophytes.
(b) Applications in Agriculture:
Obligate endophytes have an enormous potential for use because of their ability to colonize the entire plant interior and establish themselves niches protected from oxygen or other inhibitory factors; thus their potential to fix nitrogen can be expressed maximally.
Recent studies in Brazil showed that the sugarcane varieties fix up to 80% nitrogen. It has been reported that wetland rice receives some nitrogen by endodiazotrophs. Tropical pasture grasses such as Brachiaria, Digitaria, Panicum and Paspalum spp. fix nitrogen.
4. Bio-Fertilizers aiding Phosphorus Nutrition:
Tropical soils are deficient in phosphorus. Further most of the microorganisms solubilize P and thus make it available for plant growth. It is estimated that in most tropical soils, 75% super phosphate applied is fixed and only 25% is available for plant growth.
There are some fungi such as Aspergillus awamori, Penicillium digitatum, etc. and bacteria like Bacillus polymyxa, Pseudomonas striata, etc. that solubilize unavailable form of P to available form. India has 250 mt of rock phosphate deposits. The cheaper source of rock phosphate like Mussoorie rock phosphate and Udaipur rock phosphate available in our country can be used along with phosphate solubilising microorganisms (Table 34.1).
Vesicular-arbuscular mycorrhizal (VAM) fungi colonize roots of several crop plants. They are zygomycetous fungi belonging to the genera Glomus, Gigaspora, Acaulospora, Sclercystis, etc.
These are obligate symbionts and cannot be cultured on synthetic media. They help plant growth through improved phosphorus nutrition and protect the roots against pathogens. Nearly 25-30% of phosphate fertilizer can be saved through inoculation with efficient VAM fungi as reported by Bagyaraj (1992).
5. Production of Mycorrhizal Bio-Fertilizer:
Methods of inoculum production of mycorrhizal fungi differ with respects to their nature, depending upon types i.e., ectomycorrhizal or endomycorrhizal.
(i) Ectomycorrhizal Fungi:
In this case, the basidiospores, chopped sporocarps, sclerotia, pure mycelial culture, fragmented mycorrhizal roots or soil from mycorhizosphere region can be used as inoculum. The inoculum is mixed with nursery soil and seeds are sown thereafter.
Institute for mycorrhizal Research and Development, USA and Abbot Laboratories, USA have developed a mycelial inoculum of Pisolithus tinctorius in a mycelial vermiculite-peat moss substrate with trade name ‘MycoRhiz’ which is commercially available on large quantities (Table 34.2).
(ii) VA Mycorrhizal Fungi:
VA mycorrhiza can be produced on a large scale by pot culture technique. This requires the host plant mycorrhizal fungi and natural soil. The host plants which support large scale production of inoculum are sudan grass, strawberry, sorghum, maize, onion, citrus, etc.
The starter inoculum of VAM can be isolated from soil by wet sieving and decantation technique. VAM spores are surface sterilised and brought to the pot culture. Commonly used pot substrates are sand: soil (1:1, w/w) with a little amount of moisture.
There are two methods of using the inoculum:
(a) Using a dried spore-root-soil to plants by placing the inoculum several centimetres below the seeds or seedlings,
(b) Using a mixture of soil- roots, and spores in soil pellets and spores are adhered to seed surface with adhesive.
Commercially available pot culture of VA mycorrhizal hosts grown under aseptic conditions can provide effective inoculum. Various types of VAM inocula are currently produced by Native Plants, Inc (NPI), Salt Lake City.
In India, Tata Energy Research Institute (TERI), New Delhi and Forest Research Institute, Dehradun have established mycorrhizae banks. Inocula of these can be procured as needed and used in horticulture and forestry programmes.