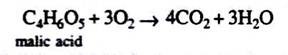The below mentioned article includes a collection of top ten experiments on respiration in plants.
1. Experiment to demonstrate the utility of oxygen in respiration:
Requirements:
A conical flask, a bent tube, germinating seeds, caustic potash in a small container, a mercury dish.
Method:
Germinating seeds are taken in a conical flask in which a container of caustic potash is also put. The mouth of this conical flask is closed by a single-holed cork through which a glass tube bent twice at right angles in inserted. The far end of this tube is put in the mercury dish. Now this apparatus is left undisturbed for some time.
Observation:
The mercury in far end of bent glass tube rises to a height of 15 cm.
Explanation:
The germinating seeds undergo aerobic respiration as they use the oxygen available inside the conical flask. As whole of the oxygen is used up by the germinating seeds, the pressure inside the flask is decreased. Hence, consequently the mercury level rises in the far end of the bent glass tube.
This level reaches only a height of 15 cm in the glass tube. As this level is about one-fifth the air. As oxygen constitutes one fifth of total composition, it is reasonable to infer that the seeds have used this gas of the air in respiration.
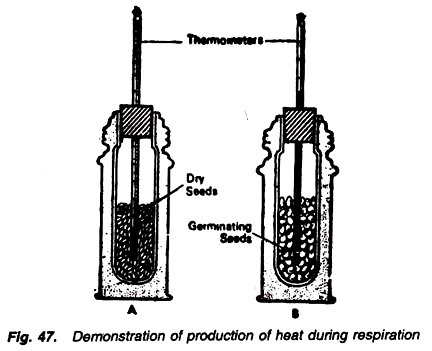
2. Experiment to demonstrate the energy is production in the form of heat during the respiration:
Requirements:
Thermos bottle (2), cork, thermometer (2), 50 germinating seeds, 50 dry seeds.
Method:
1. Take two thermos bottles fitted with a uni-holed cork.
2. In one thermos fill about 50 germinating seeds and in the other fill about 50 dry seeds.
3. Through the hole of the cork, insert a thermometer in each thermos in a way that its bulb is buried in the seeds (Fig. 47).
4. Note the initial temperature in the thermometer and keep them as such for a few hours. Note the final temperature.
Observations:
There is no change in temperature in the thermometer placed in dry seeds while the temperature rises in the another thermometer which is fitted in the thermos containing germinating seeds.
Results:
In case of the dry seeds, there is no change in the thermometer reading because they are not undergoing through the process of respiration. But distinct rise in temperature in case of germinating seeds is obviously due to the liberation of heat energy by the respiratory substrate, i.e., germinating seeds.
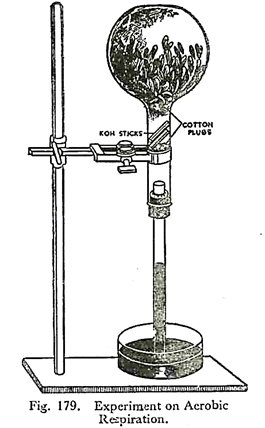
3. Experiment to demonstrate aerobic respiration:
A few germinating seeds or flower-buds are put in a flask with a cork at the mouth. Care must be taken to remove all the green parts from the flower-buds, otherwise the CO2, liberated will be at once utilised in photosynthesis.
A glass tube is fitted through the cork. Now one or two sticks of caustic potash are introduced into the flask. Two cotton plugs are put to keep the flower-buds and the caustic potash sticks in position. The flask with the tube is inverted over a trough of mercury, thus cutting off connection with external air. It is fixed in position with clamp and stand.
After a few hours it is noted that the mercury has risen in the tube covering nearly one-fifth of the total volume. The volume of mercury risen can at its maximum be one-fifth of the entire volume of the flask, for O2 in the atmosphere is only about 20%.
It is due to the fact that carbon dioxide given out during respiration has been absorbed by caustic potash producing partial vacuum. Oxygen present in the flask has been utilised for respiration. The gas that remains in the flask is nitrogen.
We know that nearly one-fifth of the volume of air is oxygen. So this experiment, besides showing intake of oxygen and giving out of carbon dioxide, also proven that volume of oxygen taken in is almost equal to the volume of carbon dioxide given out.
4. Experiment to demonstrate anaerobic respiration:
Requirements:
Germinating seeds, a test tube, mercury, KOH crystals, petri dish, etc.
Method:
The mercury is filled in the test tube up to the rim. Take a petri dish full of mercury and put the mercury filled test tube in the inverted condition with the help of thumb. The thumb is removed inside the petri dish. Now introduce some seeds (germinating or soaked) in the test tube with the help of a forceps. Let this apparatus be put as such for some time.
Observation:
After some time the mercury level in test tube goes down. Now introduce some KOH crystals in the above test tube. After a little while the mercury level will go up to the former position.
Explanation:
The lowering of mercury level in test tube indicates that some gas has been released by germinating seeds. Only CO2 gas may be absorbed by KOH crystals. We see that with the introduction of KOH crystals the level of mercury goes high, hence the seeds have released CO2 under the anaerobic conditions.
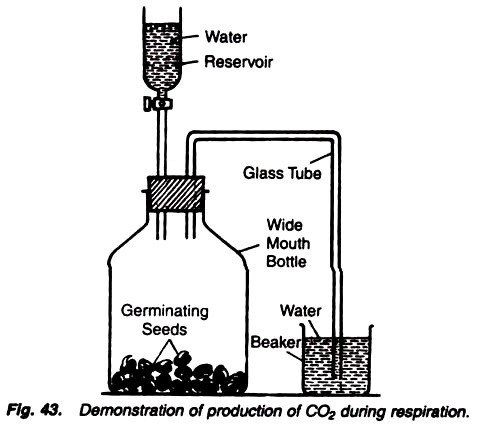
5. Experiment to demonstrate the production of carbon dioxide in aerobic respiration:
Requirements:
Bottle, germinating seeds, cork, stopper, glass tube, water, wide-mouthed tube with a stopcock, lime water.
Method:
1. Take a wide-mouthed bottled and place some germinating seeds in it.
2. Close the mouth of the bottle with a two-holed cork.
3. Insert a glass tube, bent twice at right angles, through one of the hole of cork and through the another hole insert a wide-mouthed tube functioning as water reservoir and fitted with a stopcock.
4. Dip the another end of bent glass tube into the water in a beaker (Fig. 43).
5. Keep the experiment as such for a few hours allowing the seeds to respire.
6. Now replace the water-filled beaker with a lime-filled beaker and open the stopcock of water reservoir to allow the water to go in the bottle containing seeds.
Observations:
After some time the air bubbles come out and the lime water becomes milky.
Results:
Lime water turns milky due to the carbon dioxide evolved during the process of germination of seeds. When water is poured by opening the stopcock of water reservoir, it drives out the air through the bent tube, and as the air passes through the lime water, the latter turns milky due to the fact that the air contains carbon dioxide.
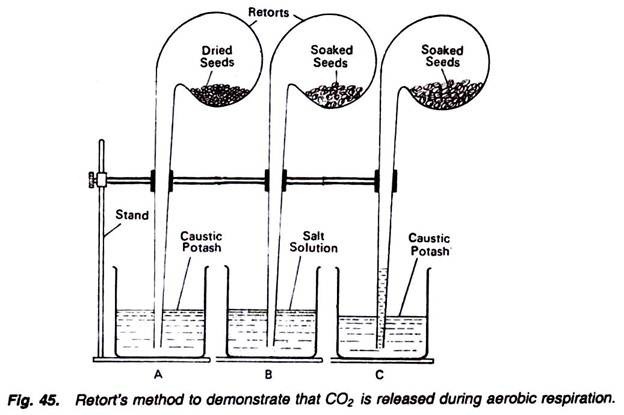
6. Experiment to demonstrate the following in plant respiration by using retort method:
i. That dry seeds to not respire;
ii. That in respiration O2 absorbed is equal to the CO2 released; and
iii. That the CO2 is produced during respiration.
Requirements:
Retorts (3), beakers (3), stands (3), soaked seeds, dry seeds, salt solution, caustic potash solution.
Method:
1. Take three retorts and connect them separately with three separate stands.
2. In the bulb of one retort, introduce dry seeds, and dip its tube in a beaker filled with solution of caustic potash (Fig. 45).
3. In the bulb of second retort, introduce soaked seeds, and dip its tube in a beaker containing salt solution.
4. In the bulb of third retort also introduce the soaked seeds and dip its tube in a beaker containing caustic potash solution.
5. Keep the apparatus as such for a few hours and observe.
Observations:
In the first and second retorts and beakers, there is no change, but in the third apparatus caustic potash solution rises in the tube of retort. Conclusions for all these three conditions can be drawn as follows:
Results:
In the first apparatus there is no change because the respiratory susbstrates, i.e., seeds, are dry and not respiring. If the respiration would have been there the CO2 released would have been absorbed by the caustic potash. Thus, ultimately the solution must have rushed into the tube of retort. So, there is no respiration.
In the second apparatus also, there is no change because the soaked seeds are respiring. They are absorbing oxygen present in the retort and producing nearly equal amount of carbon dioxide. Now because the carbon dioxide, thus released, is insoluble in salt solution, hence there will be no change.
In the third beaker the caustic potash solution rush into the retort tube. It is because of the fact that soaked seeds are respiring. They are absorbing the oxygen and releasing equal amount of carbon dioxide. The carbon dioxide thus released is absorbed by the caustic potash and thus a vacuum is created. To overcome this vacuum, caustic potash solution rushes into the tube, indicating the fact that during respiration CO2 is produced.
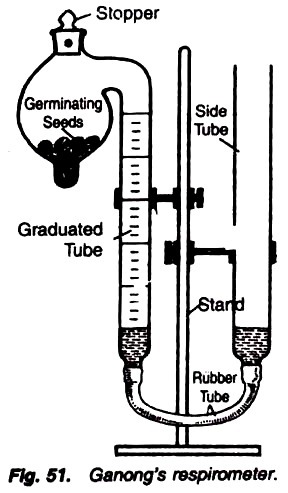
7. Experiment to determine the Respiratory Quotient (R.Q.) value of the following respiratory substrates with the help of Ganong’s respirometer:
(a) Carbohydrate
(b) Fatty seeds
(c) Organic acids
Requirements:
Ganong’s respirometer, respiratory substrate, saline water, caustic potash, stand, water, filter paper, beaker, balance with weighing box.
Method:
1. Pour some water in the lower end of the bulb of respirometer, and on putting a filter paper introduce some germinating seeds (or some other respiratory substrate in the bulb).
2. Now partly fill the respirometer with the saline water. The use of saline water is due to the fact that CO2 cannot dissolve in it (Fig. 51).
3. Twist the stopper of the bulb in such a way that the two holes come just opposite to each other. In this condition, outside air can pass into the bulbs.
4. Now adjust the level of the leveling tube and graduated tube in a way that saline is present on the same level in both the tubes.
5. Now again twist the stopper in a way that two holes are separated and the bulb is closed.
6. Note the level of the saline water and let the respiratory substrate respire for a few hours.
7. Note the final level. Introduce a caustic potash crystal and note the changes.
(A) If the respiratory substrate is carbohydrate (e.g., wheat, maize, oat, gram, pea, etc.):
Observations and results:
There is no change in the level of saline water because in the carbohydrate the volume of the O2 absorbed is equal to the volume of CO2 liberated as shown by the following equation:
CeH12O6 + 6O2 = 6CO2 + 6H2O + 673 kg cals
R.Q. = 6CO2/6O2 = 1/1 =1
The volume of the CO2 released can be estimated by adding crystals of KOH. The latter will absorb the CO2 produced by the seeds resulting ultimately into the rise of the saline level. The volume of the CO2 can be calculated by deducting the second level from the first one. Suppose it is 35 c.c.
So, R.Q. = Vol. of CO2/Vol. of O2= 35/35 = 1
(B) If the respiratory substrate is fat, (e.g., mustard or castor seeds):
Observations and results:
In case the respiratory substrate is fat, less amount of CO2 will be released than the O2 absorbed. A vacuum will be created, and to overcome this vacuum saline level will rise in the tube. This rise will be equal to the excess amount of oxygen. Denote it as V1.
It can be shown by the following equation:
Add crystals of KOH in saline solution. The level of saline again increases because KOH absorbs the CO2. A vacuum is created and to overcome it the level of saline increases. Denote it as V2.
Calculate the volume of CO2 by V2 (deducting the second rise from the first rise in level) and volume of O2 by adding both the rises in the level, i.e., V1+ V2.
So R.Q. =V2 / V1 + V2= Vol. of CO2/Vol. of O2= 102/145= 0.7
So R.Q. is less than one.
(C) If the respiratory substrate is a succulent plant (e.g., Cactus):
Observations and Results:
Succulents show incomplete oxidation in night. So, if the experiment is going on in night or if the bulb is covered with black paper incomplete oxidation of carbohydrates will be there.
Organic acids are produced in the process as shown by the following equation:
But in the day time, there will be oxidation of these organic acids as shown by the following equation:
C4H6O5 + 3O2→ 4CO2 + 3H2O
So R.Q. = 4/3 = 1.3, i.e., more than one.
(d) If the respiratory substrate is organic acid:
In this case more amount of CO2 is released in comparison to the absorbed oxygen as shown by the following equation:
So, there is an initial decrease in the level of saline water in the graduated tube. Denote it as V1. The level rises on addition of KOH solution because the latter absorbs the CO2 and thus the level increases. Denote it as V2.
So, V1 is equivalent to the excess of carbon dioxide and V2 is equivalent to the total amount of the CO2 produced. With the help of this, the volume of O2 absorbed will be V2– V1.
Thus R.Q. = Vol. of CO2/Vol. of O2= V2 / V2 – V1 = 4/3 = 1.3
8. Experiment to measure the respiratory quotient (R.Q.) by means of a pair of respiroscopes:
Requirements:
Respiroscopes (2), beakers (2), a small tube, KOH, thread, wooden stand, water, starchy seeds like maize, wheat etc., oily seeds like castor, mustard etc., thermometer.
Method:
1. Fix a pair of respiroscopes on a wooden stand.
2. In the swollen portions of the two tubes, take equal quantities of the given material, i.e., seeds.
3. A small tube containing KOH is attached in one of the tube with the help of a thread (Fig. 52).
4. Dip the lower ends of both the tubes in the water present in the beakers.
5. Fix a thermometer in the apparatus to note the temperature at which the respiration is going on in the material.
6. Keep the stopcocks of both the tubes open and suck out the air with the help of rubber tube till the water in the graduated tubes rises to a predetermined mark. Initial level in both the tubes should be the same, and note it.
7. Close the stopcock immediately and make the tubes air-tight. Wait for about an hour and note the final levels in the graduated tubes.
8. Take other respiratory substrates, i.e., fats and organic acids and repeat the experiment again as outlined above.
Observations:
In case of the carbohydrates used as the respiratory substrate, water level remains unchanged in tube A but in tube B it rises.
If the fatty seeds are used as respiratory substrate the water level rises in both the tubes.
In case of organic acids being the respiratory substrate, there is a decrease in water level in tube A while water level rises in tube B.
Results:
In case of the carbohydrates, level in tube A remains unchanged indicating that the CO2 released is equal to the O2 absorbed in respiration.
In the tube B the level of the water rises because the CO2 released is absorbed by KOH. By it, the CO2 amount can be calculated. So, R.Q. is 1.
In case of fats being the respiratory substrate, the level in the tube A increases because more amount of oxygen is absorbed and less amount of CO2 is released. To overcome this the level rises. Increase in the level of tube B is due to the CO2 released in respiration. So, R.Q. can be calculated, and it is less than one.
In case of organic acids being the respiratory substrate, the level in tube A decreases indicating the fact that in such cases more amount of CO2 is released and less amount of O2 is absorbed in respiration. Increase in level in tube B indicates the amount of CO2 released in respiration. The result in this case indicates that R.Q. is more than one because C02 released is more than the O2 absorbed in the process.
9. Experiment to demonstrate the respiratory enzymes (oxidase, peroxidase, dehydrogenase and catalase) in the plant tissues:
Requirements:
Potato, petri-dish, gum guaiacum (benzidine), alcohol, water, spirit lamp, hydrogen peroxide, 2, 3, 5- triphenyltetrazolium chloride, test tube, etc.
Method:
(A) Oxidase:
1. Cut a thin transverse section of potato with a rajor and put it in a petri-dish.
2. Immerse the section in 2% alcoholic solution of gum guaiacum (benzidine). Wait for about 15 minutes and observe the changes.
3. Take another fresh section of potato, boil it in water and repeat the same procedure mentioned above in points (1) and (2).
(B) Peroxidase:
1. Take another fresh potato section and repeat the same procedure mentioned above in points (1) and (2) for oxidase.
2. After 15 minutes remove all the gum guaiacum (benzidine) solution by adding dilute solution of hydrogen peroxide (3% H2O2 in 30 parts of water).
3. Take another fresh section of potato, boil it in water and repeat the same procedure with it also as mentioned above in points (1) and (2).
(C) Dehydrogenase:
1. To a thin potato section add 0.5% solution of 2, 3, 5, triphenyltetrazolium chloride in a petri-dish and observe the colour.
2. Take another fresh section, boil it with water, repeat the same procedure mentioned above in point (1) and observe.
(D) Catalase:
1. Take a thin potato section, add on it a dilute solution of hydrogen peroxide (1 part of H2O2 in 30 parts of water) and observe the changes.
2. Take another fresh section, boil it with water, repeat the same procedure as mentioned above in point (1) and observe.
Observations and results:
(A) Oxidase:
Development of blue colour is because of the oxidation of alcoholic solution of gum guaiacum (benzidine).
(B) Peroxidase:
Blue colour develops as in case of oxidase, but the rapidity, with which the intensity of blue colour develops, is changed. It is because in the presence of H2O2 peroxidases oxidize various substrates such as amines, phenols, etc.
Water is formed when H2O2 gets reacted with hydrogen atoms and electrons in the presence of peroxidase as under:
AH2 + H2O2Peroxidase→ A + 2H2O
where A = Hydrogen donor
(C) Dehydrogenase:
Red colour develops because of the addition of tetrazolium salt.
It is because dehydrogenases remove hydrogen atmos to oxidize the substrate. Such hydrogen atoms are received by the accepters, which thus get reduced.
(D) Catalase:
Bubbles of the oxygen are evolved.
It happens because catalase brings about decomposition of hydrogen peroxide into water as under:
2H2O2Catalase→ 2H2O + O2
10. Experiment to compare the phenomena of respiration and photosynthesis:
Requirements:
Two long-necked flasks, two mercury- containing troughs, fresh green leaves, flowers, caustic potash crystals, stands (2).
Method:
1. Take some freshly-picked green leaves and place them in a long-necked flask.
2. In the another flask place some freshly-picked young flowers.
3. With a little amount of water, moisten the leaves and flowers.
4. Invert both the flasks over separate mercury- containing troughs. Connect both the flasks with stand to hold them in a definite position and keep the whole apparatus in diffused light. (Fig. 50).
5. After about 12 hours, insert a caustic potash crystal in both the flasks and observe.
Observations:
In the flask containing green leaves there is a little rise in mercury level but sudden and large rise in mercury level is observed in the flask containing young flowers.
Results:
In the flask containing green leaves, both photosynthesis as well as respiration are going on simultaneously. The O2 evolved in the process of photosynthesis is absorbed in the process of respiration. On the contrary the CO2 released in the respiration is absorbed in the photosynthesis. Because the processes are going on in the diffused light, so the rate of photosynthesis is not very high and some carbon dioxide may accumulate in the flask and may cause a small rise in the mercury level after being absorbed by the caustic potash.
In the flask containing young flowers, sudden and large increase in the mercury level is observed because the process of photosynthesis is absent and, in the respiration process the accumulation of CO2 is continuously going on in the flask. Caustic potash crystals absorb the CO2 of the flask, causing the sudden increase in the mercury level.