Essay on Nanotechnology. The below given article will help you to learn about the following things:- 1. The Way into the Nanoworld 2. Building Blocks of Nanotechnology 3. Interaction and Topology and 4. The Microscopic Environment of the Nanoworld.
Contents
Essay # The Way into the Nanoworld:
From Micro to Nano Techniques:
Micro technology has changed our lives dramatically. The most striking impact is apparent in computer technology, which is essential for today’s industry, and also for our individual life- styles. Apart from microelectronics, micro technology influences many other areas. The size of typical structures that is accessible is in the sub-micro-meter range, which is at the limits of optical resolution and barely visible with a light microscope. This is about 1/1000 smaller than structures resolvable by the naked eye, but still 1000 times larger than an atom.
Today’s developments are addressing the size range below these dimensions. Because a typical structure size is in the nanometer range, the methods and techniques are defined as nanotechnology. The consequent extension of the resolution limit of microscopes led to instruments with the capacity to resolve features below the wavelength of light: the field ion microscope, the electron microscope, and, finally, the family of scanning probe microscopes. Now it is possible to image individual molecules, and even single atoms.
Although chemistry and micro technology appear to be fundamentally different, they are somehow related. They have mutual interests in the area of properties of materials. Micro-technology is not a simple extrapolation of conventional precise mechanical methods down to smaller dimensions.
Chemical methods—such as plasma processes, wet chemical etching and photo-resist techniques—are predominant compared with cutting or reshaping processes. However, micro technology follows physical principles.
As in classical chemistry, chemical processes in micro technology use a relatively high number of similar particles. Individual particles play no dominant role, whether in fabrication methods or in applications. In nanotechnology, the primary role of classical physical principles is replaced as molecular and atomic dimensions are approached. Physical-technical and chemical aspects influence the fabrication and the use and application of Nano-technical structures on an equal basis.
The effects of microscopic physic—a field that is influenced by and uses quantum phenomenon—complement these aspects. In contrast to classical chemistry, small ensembles or even individual particles can play a decisive role. The nanotechnology literature often focuses on the structure size and differentiates between two basic approaches.
The Top-Down approach tries to enhance the methods from micro technology to achieve structure sizes in the medium and also lower nanometer range. This approach is based on a physical and micro lithographic philosophy, which is in contrast to the other approach, where atomic or molecular units are used to assemble molecular structures, ranging from atomic dimensions up to supra-molecular structures in the nanometer range. This Bottom-Up approach is mainly influenced by chemical principles.
The challenge of modern nanotechnology is the realization of syntheses by the Top-Down and Bottom-Up approaches. This task is not driven entirely by the absolute structure dimensions, because today macro and supra-molecules extending-up to hundreds of nanometers or even micro-meters can already be synthesized or isolated from biological systems. So the overlap of both approaches is not a problem. Both techniques provide specific capabilities that can be implemented by the other. The lithographic techniques (Top- Down) offer the connection between structure and technical environment.
The interface with the surrounding system is given in this approach, but it is not really possible with the chemical (Bottom-Up) approach. At the same time, the integration of nanostructure into a functional micro-technical environment is realized. On the other hand, chemical technologies provide adjustment of chemical binding strength and preferred orientation of bonds, together with a fine tuning according to the numbers of bound atoms or atomic groups and a classification of the spatial orientation based on the number of bonds and their angles.
Therefore, nanotechnology depends on both classical micro-technology, especially microlithography, and chemistry, in particular interfacial and surface chemistry and supra-molecular synthesis. Additional basic methods are molecular biology and biochemistry, because nature has provided, with the existence of large molecules and supra-molecular complexes, not only examples, but also interesting technical tools).
Definition of Nanostructures:
A clear distinction between nanostructures and microstructures is given here, arbitrarily using length measurements. Nanostructures are defined according to their geometrical dimensions. This definition addresses technical dimensions, induced by external shaping processes; with the key feature being that the shaping, the orientation and the positioning is realized relative to an external reference system, such as the geometry of a substrate. Of less importance is whether this process uses geometrical tools, media or other instruments.
A narrow definition of nanostructures is that they include structures with at least two dimensions below 100 nm. An extended definition also includes structures with one dimension below 100 nm and a second dimension below 1 pm. Following on from this definition, ultra-thin layers with lateral sub micro-meter structure sizes are also nanostructures. All spontaneously distributed or spontaneously oriented structures in materials and on surfaces are not incorporated in Nano technical structures.
However, this does not exclude the presence of such structures in Nano technical setups, as long as their dimensions are in accord with the above-mentioned criteria. Also microstructure ultrathin layers are excluded, because they exhibit only one nanometer dimension. Nano devices are devices with at least one essential functional component that is a nanostructure. Nano systems consist of several Nano devices that are of importance to the functioning of the whole system.
Insight into the Nanoworld:
The realization that there are small things in the world that are not visible to the naked eye extends back into human history. The development of the natural sciences created an interest in the micro world, in order to enable a better understanding of the world and the processes therein. Therefore, the development of new microscopic imaging methods represents certain milestones in the natural sciences. The micro world was approached by extending the range available for the direct visualization of objects through the enhancement of microscopic resolution.
Access to spatial modifications in the Nano world is not limited to one direction. Long before instruments were available for the imaging of molecules, an understanding of the spatial arrangements of atoms in molecules and solids, in disperse systems and on surfaces had been developed.
The basis for this development was the anticipation of the existence of small building elements, which extended back to Greek philosophers (Leukip and Demokrit: ‘atomos’—the indivisible = smallest unit). ‘This hypothesis was confirmed by Dalton with the discovery of stoichiometry as a quantitative system in materials: chemical reactions are comprised of fixed ratios of reactant masses.
Based on the systematic organization of chemical elements—developed by Dobereiner, Meyer and Mendeleyev—into the Periodic Table of the elements, and supplemented by models of the internal structure of atoms, a new theory of the spatial connection of atoms was created: the theory of chemical bonds. It not only defines the ratios of atoms involved in a reaction but leads also to rules for the spatial arrangement of atoms or group of atoms. We know today that the immense variety of solid inorganic compounds and organisms is based on this spatial arrangement of chemical bonds.
Stoichiometry and geometry describe the chemical aspects of molecules and solids. The stability and the dynamics of chemical changes are determined by the rates of possible reactions that are based on thermodynamics and kinetics. Key contributions to the understanding of the energetic and kinetic foundations came from Clausius, Arrhenius and Eyring.’
Intervention into the Nanoworld:
The scientific understanding of the molecular world and the application of quantitative methods laid the foundations of modern chemistry Before the quantification of chemical reactions, there was already an applied area of chemistry, for example in mining or metallurgy. However, it was established through an empirical approach.
The understanding of the molecular context and its quantitative description, supplemented by the control of reactions by parameters derived from theoretical work or model calculations, improved dramatically the conditions for manipulations in the molecular world.
Measurements and quantitative work established the structure oriented chemistry. Synthetic chemistry, with its beginnings usually being attributed to the synthesis of urea by Friedrich Wohler (1828), provides a molecular-technical approach to the Nano world.
The formulation of binding theories and the development of analytical methods for the elucidation of the spatial arrangements in molecules (e.g., IR spectroscopy, X-ray based structure determination, and NMR spectroscopy) transformed chemistry from a stoichiometric to a structured-oriented science.
Modern chemistry is a deliberate intervention into the Nano world, because the arrangement of the bonds and the geometry of the molecules are addressed by the choice of both the reaction and the reaction parameters.
In contrast to micro technology, synthetic chemistry uses a large number of similar particles, which show a statistical distribution with regard to spatial arrangement and orientation. So today’s molecular techniques connect a highly defined internal molecular geometry with an uncertainty in the arrangement of the individual particles with respect to an external frame of reference.
Recent decades have witnessed the synthesis of an increasing variety of internal geometries in molecules and solids with small and large, movable and rigid, stabile and high-affinity molecules and building units of solid materials.
Apart from the atomic composition, the topology of bonds is of increased interest. A large number of macromolecular compounds have been made, with dimensions between a few nanometers and (in a stretched state) several micro-meters. These early steps into the Nano world were not limited to the molecular techniques. Physical probes with dimensions in the lower nanometer range are also suited to the fabrication and manipulation of nanostructures.
Essay # Building Blocks of Nanotechnology:
Nanotechnology utilizes the units provided by nature, which can be assembled and also manipulated based on atomic interactions. Atoms, molecules and solids are, therefore, the basic building blocks of nanotechnology.
However, there is a fundamental difference from the classical definition of a building material used in a conventional technical environment, which also consists of atoms and molecules in solid materials. The smallest unit in technical terms includes an enormous number of similar atoms and molecules, in contrast to the small ensembles of particles—or even individual particles—addressed in nanotechnology. This puts the definition of material into perspective.
The properties of a material are determined by the cooperative effect of a huge number of similar particles in a three-dimensional arrangement and by a mixture of only a few types of similar particles (e.g., in an alloy).
Many physical properties of materials require a larger ensemble of atoms for a meaningful definition, independent of the amount of material, for example, density, the thermal expansion coefficient, hardness, colour, electrical and thermal conductivity. With solid materials, it is known that the properties of surfaces may differ from the bulk conditions. In the classical case, the number of surface atoms and molecules is small compared with the number of bulk particles. This ratio is inverted in the case of nanoparticles, thin layers and Nano technical elements.
The properties of nanostructures are, therefore, more closely related to the states of individual molecules, molecules on surfaces or interfaces than to the properties of the bulk material. Also, the terminology of classical chemistry is not fully applicable to nanostructures. Key terms—such as diffusion, reactivity, reaction rate, turnover and chemical equilibrium—are only defined for vast numbers of particles.
So their use is limited to the case of nanostructures with small numbers of similar particles. Reaction rate is replaced by the probability of a bond change, and diffusive transport by the actual particle velocity and direction.
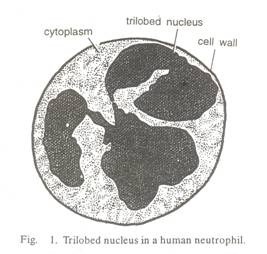
However, not all definitions from classical physics and chemistry are unimportant at the Nano scale. The consideration of single particles is preferred compared with the integral discussion of particles in solid, liquid or gaseous media. Because the dimensions extend to the molecular scale, the importance of the chemical interactions between particles is greatly enhanced compared with the classical case. Nano technical elements consist of individual particles or groups of particles with different interactions between the atoms (Fig. 1).
The following types can be distinguished:
Three dimensions for individual particles can be quite different. Atoms have diameters of about 0.1 nm; individual coiled macro molecules reach diameters of more than 20 nm. In an extended state, these molecules exhibit lengths of up to several micro-meters. In principle, there is no upper size limitation for molecules. Technical applications usually use small molecules with typical dimensions of about 1 nm besides polymers and solids with three dimensional binding networks.
Synthetic mole—Ides, such as linear polymer, exhibit, typically, molar masses of 10 000 to 1 000 000. These values correspond to particle diameters of 2-10 nm in a coiled state in most instances. Apart from the molecules, both elemental solids and compound solids are essential for nanotechnology. They are, for example, prepared as nanoparticles with dimensions ranging from a few atoms up to diameters of 0.1 pm, corresponding to about 100 000 000 atoms.
Similar values can be found in structural elements of thin atomic or molecular layers, in monomolecular films or stacks of monolayers. A number of one hundred million seems large, but it is still small compared with the number of atoms in standard micro-technological structures. This quantity corresponds to the number of atoms in individual large macromolecules, e.g. in long-chain organic polymers. It is not usually the single atom, but small solids, large individual molecules and small molecular ensembles that are the real building blocks for nanotechnology.
The nature of their connection and arrangement determines the constructive potential and functions of the Nano technical devices and systems. Besides the standard lithographic methods known from micro technology, a wide range of chemical techniques are applied in nanotechnology, from fields such as synthetic, surface, solid state, colloid and bio-molecular and bioorganic chemistry.
In addition to the importance of chemical methods in many micro lithographical processes, these methods are increasing in influence in the nanometer range to become a key component in addition to the so-called physical techniques for the creation of small structures.
Interaction and Topology:
Shaping and joining of materials to devices, instruments and machines is the prerequisite for functional technical systems. The spatial modification of material surfaces and the three-dimensional arrangement of the components result in a functional structural. This principle applies to both the macroscopic technique and the Nano world.
However, the spatial arrangement and functions at the nanometer scale cannot be described adequately by the classical parameters of mechanics and material sciences. It is not the classical mechanical parameters of solids, but molecular dimensions and individual atomic or molecular interactions (especially the local character of chemical bonds) that determine the arrangement and stability of nanostructures, their flexibility and function.
The properties of a material are controlled by the bond strengths between the particles. For shaping and joining, the processes are determined by the strength and direction of positive interactions between the joining surfaces. In classical technology and usually also in micro technology, a separation between the bonding forces in the bulk material and the surface forces has some significance. Both internal and external bonds are based on interatomic interactions, the chemical bonds.
With the dimensions of Nano technical objects approaching molecular dimensions, a combined consideration of both internal and external interactions of a material with its environment is needed. Besides the spatial separation of a material, the orientation of the internal and the surface bonds also determine the properties of materials or of material compounds.
Conventional technology uses materials with isotropic properties. Isotropic means that these properties are approximated as being similar in all spatial orientations of the solid. Restrictions are as a result of materials being created in an inhomogeneous process (e.g., wood) or materials transformed by processes inducing preferred orientation (e.g., shaping).
The macroscopic model of ideal isotropy is also not valid for single-crystalline materials such as silicon, gallium arsenide, or other typical microelectronic materials. A single-crystalline solid excludes the statistical distribution of interatomic distances and of bond orientations.
It includes elementary cells consisting of a few atoms, and a randomly oriented plane results in a density fluctuating with the angle of this plane. In addition, the bond strength between atoms is localized and is determined from its orientation. Such elementary cells create the solid in a periodic arrangement in an identical orientation. So the anisotropy of the particle density and bond strength on the atomic scale is transformed into macroscopic dimensions.
However, non-crystalline materials created by surface deposition processes can also show anisotropy almost all thin layers prepared by evaporation or sputtering exhibit anisotropy due to the preferred positioning by an initial nucleation and a limited surface mobility of the particles, which results in grain boundaries and the overall morphology of the layer.
Even spin-coated polymer layers have such anisotropic properties, because the shear forces induced by the flow of the thin film lead to a preferred orientation of the chain-like molecules parallel to the substrate plane.
The transition from an almost isotropic to an anisotropic situation is partly based on the downscaling of the dimensions. For example, a material consists of many small crystals, so these statistically distributed crystals appear in total as an isotropic material.
A classification of isotropic is justified as long as the individual crystals are much smaller than the smallest dimension of a technical structure created by the material. The dimensions of Nano technical structures are often the same as or even less than the crystal size.
The material properties on the nanometer scale correspond to the properties of the single crystals, so that they possess a high anisotropy even for a material with macroscopic isotropy. The anisotropy of a mono-crystalline material is determined by the anisotropic electron configuration and the electronic interactions between the atoms of the crystal.
It is based on the arrangement of the locations of the highest occupation probability of the electrons, especially of the outer electrons responsible for chemical bonds. The length, strength and direction of the bonds as well as the number of bonds per atom in a material, therefore, determine the integral properties of the material and the spatial dependence of these properties.
The decisive influence of number, direction and strength of interatomic bonds is even stronger for the properties of molecules. Although molecules can have symmetrical axis, outside of such axis practically all properties of the molecules are strongly anisotropic.
A material consisting of molecules can exhibit isotropic properties at a macroscopic level, as long as the orientation of the molecules is distributed statistically in all directions. At the Nano scale, anisotropy is observed, especially in the case of monomolecular layers, but also for molecular multilayers, small ensembles of molecules, clusters and individual molecules. Because Nano technological objects consist of anisotropic building blocks, it is usually not possible to construct systems where objects of the same type are distributed statistically with respect to their orientation.
On the contrary, preferred directions are chosen, and also the connection to other molecules occurs in preferred orientations. So the anisotropic connection network of smaller and larger molecules and small solids leads to a constructive network of objects and connections, with anisotropically distributed stronger and weaker bonds—both at the molecular level and in larger modules.
These networks of bonds create connection topologies, which cannot be described simply by their spatial distribution. Depending on the character of the bonds between the particles, various complex topologies can interact with each other, depending on the point of view (e.g., conductivity, mechanical hardness, thermal or special chemical stability) of the description of the connection strength.
The discussion of topological connections in three-dimensional objects at the nanometer scale assists with the evaluation of properties, which are only described in an integral manner for classical solids. These properties are essential for the function of Nano structured devices, for processes involving movement, for chemical transformations, and for energy and signal-transduction. The spatial relationship is of particular importance for the evaluation and exploitation of microscopic effects, which are unique for Nano systems, such as single quantum and single particle processes.
Essay # The Microscopic Environment of the Nanoworld:
Nanometer structures are abundant in nature and technology. The general tendency of nature towards the spontaneous creation of structures by non-equilibrium processes leads to the formation of more or less regular structures with nanometer dimensions. Such objects exist in a variety of time scales and exhibit rather dissipated or conserved character. Typical structures can be found in cosmic dust, in the inorganic structures of solidified magma, or in the early seeds of condensing atmospheric water vapour.
In contrast to many inorganic structures, the Nano-Scopic objects in Nano systems are not spatially independent, whether they are in technical systems or in natural functioning systems. They are always embedded in an environment or at least adjusted to interactions in a larger setting. Nature demonstrates this principle in an impressive manner. The smallest tools of life, the proteins, have dimensions of a few nanometers up to some tens of nanometers. They are usually found in closed compartments, in cells or cell organelles.
Often an arrangement into superstructures—as in, for example, cell membranes, can be observed. These tools for the lower nanometer range are produced in the cells as biological microsystems, and are usually also used by these cells.
The slightly larger functional Nano objects, such as cell organelles, are also integrated into this microsystem environment. The smallest biological objects with a certain functional autonomy are viruses. With dimensions of several tens of nanometers up to a few hundred nanometers they are smaller than the smallest cells; nevertheless they can connect thousands of individual macromolecules into a highly ordered and complex structure.
However, they are not able to live on their own. Only when they (or their subsystems) interact with cells in more complex Nano machinery are they able to reproduce and to induce biological effects. This principle of integrating small functional objects into a wider environment is common in technical applications.
It can already be seen in conventional construction schemes, e.g., in the combination and functional connection of several units in the hood of a car. This principle is essential in micro technology. Electronic solid state circuits combine individual electronic devices, such as wires, transistors and resistors in a chip.
The circuits are arranged on a circuit path, and these paths are assembled into machines. Approaching the nanotechnology range, even more levels of geometrical and functional integration are required, to make the Nano objects usable and the interface functional.
The large distance between the macro world with typical dimensions of centimeters to meters and the structure sizes of the Nano world has to be considered. This gap is comparable to the difference between a typical machine and up to near cosmic dimensions (Fig. 2).
The application of micro technological objects requires the integration of microchips into a macroscopic technical environment. Such an arrangement is needed to realize all interface functions between the micro and macro world. The lithographic microstructures are not accessible for robotic systems as individual structures, but only in an ensemble on a chip with the overall dimensions in millimeters.
The smallest lateral dimensions of such a structure are in the medium to lower nanometer range, but the contact areas for electrical access of the chip are in the millimeter range. This principle of geometric integration is also utilized in nanotechnology; in this case the micro technology is used as an additional interface level. Although selected nanostructures can be produced independent of micro technology, a functional interfacing of Nano systems requires the interaction with a microsystem as a mediator to the macroscopic world.
Therefore, a close connection between Nano and micro technology is required. Additionally, a variety of methods originally developed for micro technology were further developed for applications in nanotechnology.
So, not only is a geometrical but also a technological integration observed. Nevertheless, apart from the methods established in micro technology and now also used in nanotechnology (such as thin film techniques), there are other methods preferably used in only one area, e.g.: photolithography and galvanic techniques are typical methods in the micro-meter range; and scanning probe techniques, electron beam lithography, molecular films and supra-molecular chemistry are interesting methods in the nanometer range.