After reading this article you will learn about the chemical structure of chlorophyll. Also learn about the absorption spectra of chlorophyll, with the help of suitable diagrams.
A green pigment within the chloroplast (Fig. 3.40). It absorbs light energy.
Now, it is a question of interest, how these chlorophylls absorb light energy? Chlorophyll traps the light energy, hence called as photoreceptor. It is the green pigment found in plants. The basic structure of a chlorophyll molecule is a porphyrin ring, coordinated to a central atom. This is very similar in structure to the heme group found in hemoglobin, except that in heme the central atom is iron, whereas in chlorophyll it is magnesium. Light absorbed by chlorophyll excites the electrons in the ring.
Different wavelengths of light excite the electrons by different amounts. Chlorophylls are very effective photoreceptors because they contain a network of alternating single and double bonds and the orbitals can delocalize the electrons and stabilize the structure. Such delocalized polyenes have very strong absorption bands in the visible regions of the spectrum, allowing the plant to absorb the energy from sunlight.
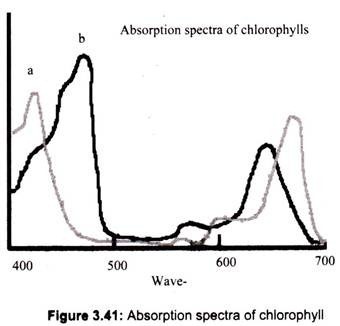
There are mainly two types of chlorophyll, named a and b, which differ in the composition of a side chain (in a it is -CH3, while in b it is CHO). Chlorophyll a, gives absorption peaks at 430 nm and 662 nm, while Chlorophyll b gives peaks at 453 nm and 642 nm. The different side groups in the two chlorophylls ‘tune’ the absorption spectrum to slightly different wavelengths.
Thus, these two kinds of chlorophylls complement each other in absorbing sunlight. For instance, the light which is not significantly absorbed by chlorophyll a, at 460 nm, will be strongly absorbed or captured by chlorophyll b. The plants can obtain all their energy requirements from the blue and red region of the spectrum, however, there is still a large spectral region, between 500 and 600 nm, where very little light is absorbed (Fig. 3.41). This light is in the green region of the spectrum, and since it is reflected, this is the reason why plants look green.
The energy in the ‘excited electrons’ can be passed from one chlorophyll molecule to another, but at the end it will just be lost as fluorescence (i.e. the energy will be re-emitted as light), unless the excited electron itself can be ejected from the chlorophyll molecule. There are two different sorts of reaction centers in plants. In each of these reaction centers, the ejected electron is transferred to an acceptor molecule, which can then pass it on to a different molecule and eventually the electron(s) can be used to fix up carbon dioxide. However, it is not feasible to keep on ejecting electrons from these chlorophyll molecules, electrons must be fed back in to the system to replace those which have been ejected. These electrons come from water, resulting in oxygen being evolved. Thus, this reaction is a non-cyclic electron transport chain.