This article throws light upon the five assays developed for measuring the cell viability and cytotoxicity.
The five assays are: (i) Cytotoxicity and Viability Assays (ii) Survival Assays (iii) Metabolic Assays (iv) Transformation Assays and (v) Inflammation Assays.
As the cells are removed from the living (in vivo) environment and subjected to experimental manipulations in the culture systems (in vitro), their viability assumes significance. Viability of the cells represents the capability of their existence, survival and development.
Many experiments are carried out with cells in the culture rather than using the animal models. This is particularly so with regard to the determination of safety and cytotoxicity of several compounds (pharmaceuticals, cosmetics, anticancer drugs, food additives). In vitro testing for cytotoxicity and safety evaluation is in fact cost-effective, besides reducing the use of animals.
Studies on cytotoxicity broadly involve the metabolic alterations of the cells, including the death of cells as a result of toxic effects of the compounds. For instance, in case of anticancer drugs, one may look for death of cells, while for cosmetics the metabolic alterations and allergic responses may be more important.
There are several assays developed in the laboratory for measuring the cell viability and cytotoxicity.
They are broadly categorized into the following types:
i. Cytotoxicity and viability assays.
ii. Survival assays.
iii. Metabolic assays.
iv. Transformation assays.
v. Inflammation assays.
i. Cytotoxicity and Viability Assays:
A majority of the cytotoxicity and viability assays are based on the measurement of membrane integrity, cellular respiration, radioisotope incorporation, colorimetric assays and luminescence- based tests.
Based on membrane integrity:
The most common measurements of cell viability are based on membrane integrity. The damage to membrane may occur due to cell disaggregation, cell separation or freezing and thawing. Membrane integrity can be determined by uptake of dyes to which viable cells are impermeable (e.g. naphthalene black, trypan blue, erythrosin) or release of dyes normally taken up and retained by viable cells (e.g. neutral red, diacetyl fluorescein).
The other assays for membrane integrity are release of labeled chromium (51Cr), enzymes and use of fluorescent probes. Cell viability measurements, based on membrane integrity are immediate that can be detected within a few hours. However, these measurements cannot predict the ultimate survival of cells.
Dye exclusion assay:
The principle of this assay is based on the fact that viable cells are impermeable to several dyes such as naphthalene black, trypan blue, eosin Y, nigrosin green and erythrocin B. The technique basically consists of mixing the cells in suspension with the dye and examining them under the microscopy. The stained cells and the total number of cells are counted. The percentage of unstained cells represents the viable cells.
Dye exclusion assay is convenient and suitable to suspension cultures than to monolayers. This is due to the fact that as the dead cells detatch from the monolayers they are lost from the assay. The major limitation of this assay is that reproductively dead cells do not take up the dye, and will be counted as though they are viable.
Dye uptake assay:
The viable cells can take up the dye diacetyl fluorescein and hydrolyse it to fluorescein. The latter is held up by the viable cells, as it is impermeable to membrane. The viable cells therefore emit fluorescein green while the dead cells do not. Thus, the viable cells can be identified.
Labeled chromium uptake assay:
Labeled chromium (51Cr) binds to the intracellular proteins through basic amino acids. When the cell membrane is damaged, the labeled proteins leak out of the cell and the degree of leakage is proportional to the amount of damage. Labeled 51Cr uptake method is used in the immunological studies to determine the cytotoxic activity of T-lymphocytes against target cells.
Enzyme release assays:
The membrane integrity of cells can also be assessed by estimating the enzymes released. Lactate dehydrogenase (LDH) has been the most widely used enzyme for this purpose.
Based on cellular respiration:
Respiration of the cells measured by oxygen utilization or carbon dioxide production can be used to assess cell viability. This is usually done by using Warburg manometer.
Based on radioisotope incorporation:
By using radiolabeled substrates or metabolites, the radiolabel in the products formed can be detected. This method is particularly useful for the cytotoxicity assays of drugs. Some of the important radioisotope incorporation methods are briefly given.
Labeled nucleotides:
Incorporation of (3H) thymidine into DNA and (3H) uridine into RNA are widely used for the measurement of drug toxicity.
Labeled phosphate:
The cells are pre-labeled with 32P. When the damage occurs to cells, they release labeled phosphate which can be measured. The efficacy of drugs can be evaluated by this approach.
Based on colorimetric assays:
The recent developments in the colorimetric assays by using sophisticated micro-plate readers are fruitfully utilized for quantitation of cells. A good correlation between the cell number and colorimetric assay are observed.
Some highlights of this approach are given below:
i. Protein content can be estimated by methylene blue, amido black, sulforhodamine. In the Lowry method for protein estimation, Folin-Ciocalteau reagent is used.
ii. DNA can be quantitated by staining with fluorescence dyes e.g. 2-diaminodino- phenylindone.
iii. Lysosomal and Golgi body activity by using neutral red.
iv. Enzyme activity assays e.g. hexosaminidase, mitochondrial succinate dehydrogenase.
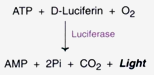
Based on luminescence test:
The viability of cells can be measured with good sensitivity by estimating ATP levels by luminescence based test. The principle is based on the following reaction.
A good sensitivity of this test is reported for the cells in the range of 20 to 2 × 107 cells/ml.
Based on apoptosis:
Most of the anticancer drugs kill cells by apoptosis which can be measured for the assessment of cytotoxicity. Apoptosis can be detected by the following ways.
i. Changes in the morphology.
ii. Detection of phosphatidyl serine in the membrane by using annexin V conjugated to fluorescein isothiocyanate (FITC) or biotin.
iii. DNA laddering.
ii. Survival Assays:
The tests described above for measurement of cell viability and cytotoxicity are short-term, and they identify the dead/live cells at the time of assay. Many times, when the cells are subjected to toxicity (i.e. exposed to drugs, irradiated), the effects are not immediate, but may be observed after several hours or sometimes even days. The assays based on the survival of cells (i.e. retention of regenerative capacity or reproductive integrity) are preferred.
Clonogenic assay:
In clonogenic assay, the survival of the cells is measured by plating efficiency (i.e. the percentage of cells seeded at subculture that give rise to colonies). The plating efficiency measures the proliferative capacity for several cell generations.
Clonogenic assay broadly consists of the following stages:
1. Treatment of the cells with varying concentrations of experimental agent for about 24 hours.
2. Trypsinization followed by seeding of cells at low density.
3. Incubation of the cells for 1-3 weeks.
4. Staining and counting of the colonies.
A survival curve (semi-log plot) representing the survival fraction of the cells against drug concentration is depicted in Fig 38.1. The inhibitory concentration (IC) refers to the drug concentration required to inhibit the viability of cells. Thus, IC50 and IC90 represent the concentrations of a compound that respectively inhibit 50% and 90% of colony formation.
As is evident from the graph, the curve has a knee wherein IC50 lies, while IC90 falls in the linear range. Therefore, the differences will be more significant in the linear range.
The clonogenic assay is influenced by several factors, the important ones are listed:
i. Concentration of the toxic agent.
ii. Duration of exposure.
iii. Cell density during exposure.
iv. Cell density during cloning.
v. Size of colony.
MTT-based cytotoxicity assay:
The tetrazolium salt 3, (4.5-dimethyl-thiazol- 2-yl)-2, 5-diphenyl tetrazolium bromide) is commonly known as MTT. It is dye, and is widely used in cytotoxicity assays. The growing cells in the log phase are exposed to cytotoxic drug. The drug is then removed and the cells are allowed to proliferate for 2-3 population doubling times (PDTs). The number of surviving cells can be detected by MTT dye reduction. The concentration of MTT-formazan formed can be determined spectrophotometrically.
MTT-based cytotoxicity assay is carried out in the following stages:
1. Incubation of monolayer cultures with varying drug concentrations in micro-titration plates.
2. Removal of drug and feeding of plates to achieve 2-3 PDTs.
3. Treatment of plates with MTT, and removal of medium and MTT.
4. Measurement of MTT-formazan in an ELISA plate reader.
When the absorbance of test wells/control wells of the micro-plate is plotted against the concentration of the cytotoxic drug, a sigmoid curve is obtained.
iii. Metabolic Assays:
The metabolic assays are based on the measurements of metabolic responses of the cells. These test are carried out after exposure of the cells to cytotoxic drugs (either immediately or after 2-3 population doublings). The most commonly used metabolic measurements are DNA, RNA or protein synthesis (by estimating their concentration), besides the assay of certain dehydrogenase enzymes.
Limitations of metabolic assays:
The estimation of the total content of DNA protein may or may not be indicative of increase in cell number. This is because these assays cannot discriminate between the proliferative and metabolic activity of cells. Some workers therefore prefer to confirm the metabolic measurements by colonogenic survival assay.
iv. Transformation Assays:
The following are the commonly used assays for measurement of in vitro transformation:
i. Evidence of mutagenesis.
ii. Anchorage independence.
iii. Reduced density limitation of cell proliferation.
Mutagenesis:
Mutagenesis can be assayed by sister chromatid exchange (SCE). SCE basically involves the reciprocal exchange of DNA segments between sister chromatids at identical loci in the S-phase of cell cycle. Sister chromatid exchanges are more sensitive to mutagenesis than chromosomal breaks. For this reason, SCEs are preferred in mutagenesis research and transformation assay. The SCE technique basically involves the incorporation of radioactive nucleotides into replicating DNA and detection of SCEs by fluorescence plus Giemsa (FPG) technique.
v. Inflammation Assays:
Inflammation assays are required for testing the various forms of allergy induced by cosmetics, pharmaceuticals and other xenobiotic. These assays are at the early stages of development in the culture cells.