In this article we will discuss about:- 1. Types of Chromosome Pairing 2. Factors Affecting Chromosome Pairing 3. Significance 4. Molecular Basis.
Contents
Types of Chromosome Pairing:
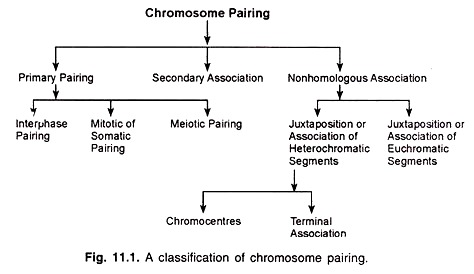
Chromosome pairing may be divided into three main types, viz., primary pairing, secondary association and non-homologous association, which may be further classified (Fig. 11.1).
Primary Pairing:
Primary pairing is observed at interphase, mitosis and meiosis. It involves the synapsis of chromosomal regions which are genetically homologous.
(a) Interphase Pairing:
During interphase, homologous chromosomes in the salivary gland cells of the Dipteran larvae remain paired lengthwise. They undergo 8 to 9 cycles of replication (endomitosis) and form the polytene giant chromosomes which have 1024 to 2048 strands.
Pairing occurs throughout the entire length of the chromosomes; this leads to the appearance of specific and constant patterns of transverse bands. The condensed bands (densely stainable) are called chromosomes.
(b) Mitotic or Somatic Pairing:
This type of chromosome pairing is found in the cells undergoing mitosis for the development of the germ line, gonads and also of the soma. The homologous chromosomes in the diploid cells are closely apposed in the prophase stage but their association at metaphase is rather loose.
Mitotic pairing differs from meiotic pairing. When more than two homologues are present, such as, in polysomics and auto-polyploids, all the homologous chromosomes are paired throughout their length.
On the other hand, the meiotic pairing is always two-by-two, i.e., it is always restricted to only two chromosomes at any point even when more than two homologous chromosomes are present. Mitotic pairing is found in the prophase and metaphase stages in the members of order Diptera.
(c) Meiotic Pairing:
This type of pairing occurs in the cells undergoing meiosis. Synapsis occurs between the homologous chromosomes throughout their length in the zygotene-pachytene stage of prophase I. Pairing in initiated near the ends of chromosomes and proceeds in a zipper like fashion.
In 1966, Sybenga proposed that there may be specific loci (sites) called zygomeres where pairing is initiated. The zygomeres may be present either in large or small numbers along the chromosomes. There may be multiple initiation sites of pairing.
In the tetraploid cells of locust (Schistocerccigregaria) a minimum of six independent sides have been found at which synapsis begins between the homologous chromosomes. The meiotic pairing is strictly homologous (gene-to-gene pairing) and it always involves only two chromosomes at any given point. At the end of pachytene, pairing affinity between homologues is lost. After pachytene, i.e., at diplotene, diakinesis and MI, the homologous chromosomes are held together by chiasmata (chiasmate meiosis).
In certain species of plants and animals, chiasmata are not formed and the homologous chromosomes are associated by a persistent pairing force during the spindle stage of metaphase I; such type of meiosis is called achiasmate meiosis.
Generally, this type of meiosis occurs in male meiocytes. Examples are PMCs of plant species Fritillaria (hermaphrodite). Achiasmate meiosis in animal species is found in males of Drosophila, Caliimcntisantillarum (mantids), Panorpa, Mecoptera, Tipula and Phryne etc.
It has been reported to occur in both sexes in hermaphrodite worms Buchholziafallax and Marionina. Association of homologues at later stages is maintained by lateral attraction or terminal attraction in such cases.
Noda in 1975 summarised the characteristics of achiasmate meiosis in Fritillaria japonica group in PMCs as follows:
(i) Synapsis of homologous chromosomes is prolonged up to MI and the association is maintained by lateral attraction,
(ii) Separation of non-sister chromatids is suppressed and thus there is no typical diplotene and diakinesis.
(iii) Homologous chromosomes apposed in parallel are usually devoid of chiasmata at MI.
(iv) Separation of homologues is initiated at the kinetochore region at early MI and progresses towards the distal ends.
Electron microscopic studies have shown that a synatinemal complex (also, synaptonemal complex) is formed when the homologous chromosomes undergo pairing.
Secondary Association:
Secondary association is observed in some polyploid species which form bivalents. In these species, genetically similar (homologous) bivalents are often found in pairs. This type of association occurs after the lapse of primary pairing and therefore, it is called the secondary association.
It has also been found that the bivalents which are genetically similar may lie close to one another on the equatorial plate more frequently than that expected for random distribution. However, this type of association has no effect on the segregation of chromosomes. Secondary association is used as a criterion of remote polyploidy.
Non-homologous Association:
There are two main types of non-homologous association, one related to the heterochromatin and the other related to the euchromatin.
(a) Association of heterochromatic segments:
(i) Chromocentres:
Heterochromatin has a tendency to fuse with other heterochromatic entities. The formation of chromo Centre in the interphase nuclei is an example of this type of pairing. Chromocentres consist of heterochromatin and are positively heteropycnotic.
Chromocentres are variable in size and irregular in occurrence; these suggest the involvement of a surface adhesion or stickiness phenomenon in their formation. Centromeric Chromocentres are formed in many Dipteran nuclei possessing polytene chromosomes. Thus this type of pairing involves lateral or terminal juxtaposition or fusion of heterochromatic segments.
(ii) Terminal association:
This type of pairing is observed at MI of meiosis in some cases. It refers to the terminal associations between univalent chromosomes, which may be due to heterochromatic fusions.
(b) Association of euchromatic chromosome segments:
This type of association of non-homologous chromosomes occurs during meiosis in zygotene and pachytene stages. Such pairing may also lead to chiasma formation. This may indicate some duplication in the genome. In mono-haploids, non-homologous pairing has been observed in some plants, such as, maize, barley and Antirrhinum majus etc.
Factors Affecting Chromosome Pairing:
Chromosome pairing is influenced by several genetic and environmental factors which are briefly described below.
A. Genetic Factors:
Genetic control of pairing operates at two levels:
(i) Quantitative and
(ii) Qualitative levels.
(i) Quantitative genetic control:
There are two extremes regarding chromosome pairing, asynapsis and de-synapsis. In case of a synapsis, pairing does not occur between homologous chromosomes and univalents are formed at prophase I and MI.
On the other hand, in de-synapsis chromosomes do pair during zygotene-pachytene, but they fail to remain paired in the subsequent stages due to a lack of chiasma formation, resulting in univalents at diplotene, diakinesis and MI. De-synaptic mutants may be abnormal in the formation of their synaptinemal complexes.
Both these phenomena lead to the occurrence of univalents at MI. There may be many grades between these two extremes. For instance in wheat (T. aestivum), the presence of univalents in different lines and their presence in crosses and back crosses is under polygenic control; the frequency of univalents increases in wheat plants lacking chromosome 3B.
Thus the presence or absence of this chromosome affects the univalent frequency and pairing behaviour. If two rye (Secalecereale) chromosomes are added to the wheat complement, the pairing of rye chromosomes is reduced. Thus interaction of wheat and rye genotypes influences the pairing behaviour of rye chromosomes.
(ii) Qualitative genetic control:
Deviations from the normal chromosome pairing and chiasma formation have been mentioned before. Asynapsis and de-synapsis both are known to be governed by single recessive genes, although in several cases, environmental factors also play role in these two phenomena. Based on the action of the genes and univalent formation, asynapsis and de-synapsis may be weak, medium-strong or strong.
An interesting example of qualitative genetic effect on chromosome pairing comes from the bread wheat (T. aestivum). This species is an allohexaploid with the genomic constitution AA BB DD, the basic chromosome number (x) being 7.
Thus there are 7 groups of 3 pairs of homoeologous chromosomes, but 21 bivalents (21II) are formed regularly. But in nulli-5B plants, pairing between homoelogous chromosomes does take place as a result of which, multivalents are also formed.
A dominant gene (Ph) located in the long arm of the chromosome 5B (= 5BL) distal to the centromere prevents the pairing between homoeologous chromosomes. Further, when this gene is mutated, there occurs multivalent formation due to homoeologous pairing.
The gene on 5B chromosome of wheat is hypostatic to that present in the wild type 5B. When the genome BB of Aegilopsspeltoides is introduced into wheat through hybridization, the action of single remaining 5B chromosome of wheat is suppressed by the Aegilops 5B chromosome; as a result, pairing occurs between homoeologous chromosomes.
It has been reported that 5BL gene suppresses somatic pairing during the pre-meiotic mitosis. This reduces the attraction between the homoeologous chromosomes so that they do not come together for meiotic pairing. Interactions also occur between the different systems related to chromosome pairing. In wheat, absence of 3B causes a reduction in pairing, whereas the absence for 5B leads to an increase in chromosome pairing.
Absence of both 3B and 5B generally leads to a mosaic of their expression. For example, hybridization between T. aestivum different for both 3B and 5B and Secalemontanum results in their separate expressions in different cells of the same anther. Most of the cells express the effects of the deficient condition of 5B, while some (16%) cells express the results of deficient condition of 3B.
B. Environmental Factors:
(i) Temperature:
Temperature is the most important factor affecting chromosome pairing. It influences the polytene chromosome pairing during interphase as well as the prophase pairing in chiasmate meiosis. Both low and high extremes of the temperature cause a reduction in chromosome pairing during zygotene, both in plant and animal species.
(ii) Chemicals:
Chromosomes pairing is also affected by certain chemicals, such as, colchicine. Colchicine reduces chromosome pairing as a result of which, recombination is also reduced.
Significance of Chromosome Pairing:
A. Synapsis during zygotene-pachytene stage brings the homologous chromosomes close together. Cytological and genetic crossing overs occur during the period when the chromosomes are synapsed. After the occurrence of crossing over, attraction between the homologous chromosomes is apparently lost and they are held together only by the chiasma (ta).
B. For proper co-orientation and segregation of the homologous chromosomes, pairing is absolutely essential. In a chiasmate meiosis, the function of pairing is to maintain the bivalent association for proper co-orientation and segregation; this type of pairing persists up to ML.
C. Interphase pairing (polytene chromosomes) and mitotic pairing (somatic pairing) of chromosomes may facilitate the diffusion and transfer, from one homologous to another, of chromosome-limited substances involved in the regulation of genaction.
Molecular Basis of Chromosome Pairing:
A new era in the studies of chromosome pairing was ushered in with the introduction of electron microscope to biological research. Working with the spermatocytes of crayfish, Moses in 1956 discovered a tripartite ribbon at the site of synapsis; this structure is called synaptonemal complex or synaptinemal complex.
This complex occupies the space between the paired homologous chromosomes and appears to be a advice to bring together and allign the DNA molecules from paired homologous chromosomes which facilitates crossing over between them.
Synaptinemal Complex:
Synaptinemal complexes have been detected in almost all the eukaryotic cells undergoing meiosis. Synapsis starts at the beginning of zygotene when synaptinemal complex begins to develop between the homologous chromosomes. Synapsis is preceded by the formation of a rope-like proteinaceous axis along each of the homologous chromosomes.
As pairing advances, the axes of homologous chromosomes appear to adhere to each other to become the lateral elements of the synaptinemal complex. These lateral elements of the two chromosomes together form a third band called the central element (Fig. 11.2).
The formation of the central element is preceded by the development of protein connections between the chromosomes undergoing synapsis. Close association of chromosomes is found to start at the chromosome ends attached to the nuclear membrane. The three bands (two lateral and one central) from the synaptinemal complex.
Synaptinemal complexes vary in size and organization in different eukaryotic cells. In tomatoes, the overall width of the complex is approximately 160 nm, whereas in mammals, and crickets it is 180 nm and 200 nm, respectively.
The lateral elements may be spaced from 20-30 nm to 100-125 nm. The central element generally ranges from 12 to 20 nm in diameter; it is separated from the lateral elements by a space of about 40 nm.
The presence of the central element fibres of about 2 nm diameter is revealed in cross sections (Fig. 11.2). The two lateral element seem to be composed of granules and fibres (synaptomeres) that are slightly wider than 10 nm.
The major part of synaptinemal complex is protein as revealed by the cytochemical analyses. The lateral elements contain a small amount of DNA which is thought to be derived from the chromatids with which they are associated.
DNA is also present in the transverse fibres that cross the space between the central and lateral elements. It seems that the synaptinemal complex is made up of a framework of protein within which DNA containing chromosomal fibres are interspersed.
After pachytene, the synaptinemal complex dissolves and the homologous chromosomes begin to de-synapse; this stage is called diplotene during which homologues are associated by chiasmata. Synaptinemal complex may not get fully dissolved in some cells and its remnants may persist through the second meiotic division and form aggregates called poly-complexes.
Synaptinemal complex brings the homologous chromosomes close together so that crossing over may occur between them. In D. melanogaster females homozygous for the mutant gene c3G, genetic crossing over is suppressed and synaptinemal complex does not form. Similarly, crossing over does not occur in male Drosophila and synaptinemal complex also is absent.
Synaptomere-Zygosome Hypothesis of the Formation of Synaptonemal Complex:
This hypothesis states that homologous chromosomes are attached to the nuclear membrane with their ends and that two substances, namely, synaptomere (made up of polysegments) and zygosomes (composed of protein molecules) are involved in chromosome pairing, as shown by King in 1970.
Synaptomeres are distributed along the length of synapsed chromosomes; each synaptomere consists of three segments designated as A (lateral), B (central) and C (lateral segments arranged in the following order, ABC, CBA, ABC, CBA, …. (Fig. 11.3). The lateral segment A pairs with A and C pairs with C of the same chromosome, and it causes the chromosome to become shorter and thicker due to folding.
The B segment of the synaptomere is directed towards the central element and acts as the site for zygosome attachment. The zygosome is rod-shaped subunit and its one end (head) is attached to the B segment, while the other end (tail) is attached to the tail of the other zygosome attached to the homologous chromosome generating a ladder-like arrangement. The tails of B segment may be charged sites.
The mechanism and force due to which the homologous chromosomes become precisely aligned for the paring are not exactly known. Some of the investigators are of the view that the homologous chromosomes are prepared for synapsis by the attachment of their telomeres to the attachment sites on the nuclear membrane.
There is an increase in the content of “colchicine binding protein” during the leptotene-pachytene period; this increase may have some role in synapsis.
Recombination Nodules:
The occurrence of certain modifications in the synaptinemal complex at the crossover sites has been observed. These modifications have been called “nodes” or “recombination nodules” by Carpenter in 1975; they are considered to be involved in the active process of recombination.
Recombination nodules are approximately 90 nm in diameter and may be of varying shapes, such as, spherical, ellipsoidal or bar-like, and are composed of protein. They are placed between the homologous chromatids on the synaptinemal complex.
Recombination nodules are considered to mark the site of multi-enzyme recombination machine that brings local region of DNA on the maternal and paternal chromatids together across the synaptinemal complex, as suggested by Alberts et al. in 1983.
The available indirect evidences for the function of recombination nodules in recombination is summarised below:
(i) Total number of recombination nodules is about equal to the number of chiasmata observed during prophase I.
(ii) The distribution pattern of recombination nodules and crossover events along the synaptinemal complex are comparable,
(iii) Certain mutants of Drosophila show reduced recombination frequencies; these mutants also exhibit relatively fewer recombination nodules,
(iv) The radioactive DNA precursors are known to be preferentially incorporated into pachytene DNA at or near the recombination nodules.