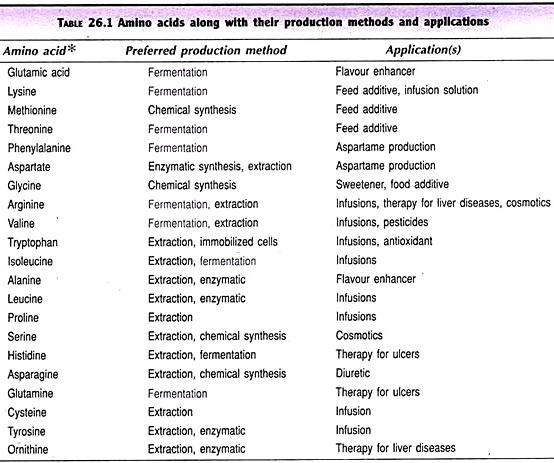
Here is a compilation of notes on Cell Cycle. After reading these notes you will learn about: 1. Introduction to Cell Cycle 2. Phases of Cell Cycle 3. Cell Cycle Checkpoints 4. Cell Cycle Regulation in Yeast.
Note # 1. Introduction to Cell Cycle:
Growth of a plant involves division of cells along with other associated aspects of metabolism. The progression from one cell division to the next is a cyclic process, the cell cycle, representing the period between two consecutive divisions.
During the time the cell must replicate its contents and then organize the distribution of its components equally between two daughter cells. The duration of cell cycle varies greatly from one cell to another, e.g., in embryonic cells it lasts for few minutes, in Allium cepa it is approximately 12 hours, sometimes it may be more than 24 hours.
Note # 2. Phases of Cell Cycle:
The cell cycle is divided into two fundamental parts — interphase and dividing phase.
Interphase:
Interphase is the period from the end of one cell division to the start of the next division. The term resting phase is sometimes used for this phase, which is a misnomer, more accurately called metabolic phase. The phase is characterized by high rate of metabolism involving both protein and nucleic acid metabolism. In this phase the cell becomes enlarged in size due to high growth rate.
Interphase constitutes the longest period of the cell cycle and is sub divided into three successive phases — G1, S and G2. The S or synthetic phase is the intermediate phase between G1 growth phase (gap-1) and G2 growth phase (gap-2).
The replication of DNA is accomplished during S phase. In G1, cellular metabolic rate is high and synthesis of mRNA, tRNA, rRNA and proteins occur, intensive cellular synthesis also occurs in G2 phase. G2 is followed by divisional phase (Fig. 5.1).
Divisional Phase:
Divisional phase or M phase involves the division of the nucleus (karyokinesis) and the division of cytoplasm (cytokinesis). The cell division in plant kingdom, specially in eukaryotes, are of two types – Mitosis and Meiosis. Mitotic division occurs in somatic or body cells and is responsible for maintaining the constancy in chromosome number in all cells of an individual.
The meiosis on the other hand, occurs in reproductive organs and is responsible for the formation of gametes or spores after halving the chromosome number. The former is also known as equational or homotypic, whereas the latter is termed as reductional or heterotypic cell division.
A regular cell cycle exists only for growing cells, the differentiated cells do not enter into S phase, stopped dividing and arrested in non-cycling state called G0 state (Quiscent phase). They have indefinitely withdrawn from the cell cycle or may remain arrested temporarily and in response to some stimulus re-enter into G1 phase.
Successive S phases without entering into divisional phase leads to endopolyploidy through endomitosis.
Note # 3. Cell Cycle Checkpoints:
The sequential events of the cell cycle are directed by a distinct cell cycle control system. The signals are transmitted within the cell by signal transduction pathways. Cells have their built in stop signals that halt the cell cycle at checkpoints until overridden by go-ahead signals. A checkpoint in the cell cycle is a critical control point where stop and go-ahead signals regulate the cycle.
Many signals registered at checkpoints come from cellular processes and sometimes the checkpoints register signals from outside the cell.
There exists a series of checkpoints which refer to the points of monitoring of cell cycle events such as DNA replication, DNA damage repair, spindle assembly, congression of chromosomes and separation of chromatids/chromosomes to opposite poles, generating signals in case of errors in processes and halting the cell cycle at specified points.
Thus in the cell cycle there are three main types of checkpoints:
(i) DNA damage checkpoint,
(ii) DNA replication checkpoint,
(iii) Spindle checkpoint.
A large number of cell cycle mutants have been isolated for these checkpoints.
In the cell cycle, checkpoints have been identified at three stages (Fig. 5.24A):
(i) G1 checkpoint:
Transition from G1 phase (start),
(ii) G2 checkpoint:
Transition from G2 to M phase,
(iii) M-phase checkpoint:
Within mitosis/meiosis itself.
Recent studies indicate the presence of four checkpoints in cell cycle as explained in Fig. 5.24B.
G1 Checkpoint (DNA Damage Checkpoint):
It is also called the ‘restriction point’ and is most important in the cell cycle start phase. If the cell receives a go-ahead signal at the G1 checkpoint, it usually completes the cell cycle and divides. If it does not receive the go-ahead signal, the cell exits from the cell cycle and switches to a non- dividing state, the G0 phase.
This checkpoint monitors damaged DNA which detects DNA damage and does not allow entry of cell into S phase until the damage is repaired.
This checkpoint blocks progression into S phase by inhibition of S-Cdk complex. Damaged DNA stimulates transcription of many genes which encode the proteins that bind to S-Cdk, inhibits their activity and thus blocks the entry into mitosis.
In mammals, a protein of p53 gene causes delay in entry of cells with damaged DNA into S phage and mutation in p53 gene, therefore, causes cancer due to increase in frequency of cancer promoting genetic alterations. The protein p53 blocks the activity of Cdks and has been dubbed as ‘Watchman’ because DNA damage is sensed by it (Fig. 5.25a).
G2 Checkpoint (DNA Replication/DNA Damage Checkpoint):
It is the controlling point involved in driving the cell to the M phase. This is triggered by MPF (Maturation Promoting Factor or M-phase Promoting Factor) which is a cyclin-Cdk complex (Cdk – cyclin dependent kinase). It promotes mitosis by phosphorylating a variety of other protein kinases.
This checkpoint monitors un-replicated and damaged DNA which delays mitosis until DNA replication is complete and DNA damage is repaired. Mitotic entry of G1 cells is delayed in yeast by the rad 9 gene.
The damaged DNA sends a signal to a series of protein kinases that blocks the dephosphorylation and activation of M-Cdk, blocking entry into mitosis (Fig. 5.25b). Normal cells treated with hydroxyurea, inhibitor of DNA synthesis, activates this checkpoint mechanism that arrests the cells in S phase, thus delaying mitosis.
M-phase Checkpoint (Spindle Attachment Checkpoint):
It ensures that ail the chromosomes are properly attached to the spindle at the metaphase plate before anaphase. A signal to delay anaphase originates at kinetochore which inhibits attachment of spindle microtubule, this keeps the anaphase promoting complex (APC) in an inactive state.
After attachment of all kinetochores, the APC is activated, triggering breakdown of cyclin and inactivation of proteins holding sister chromatids together. Mutants in MAD and BUB genes of mammals and yeast inactivate this spindle checkpoint (Fig. 5-26); The effect of colchicine which inhibits spindle assembly demonstrate the presence of this checkpoint.
Note # 4. Cell Cycle Regulation in Yeast:
Regulation of cell cycle in yeast has been studied extensively — both in fission and budding yeast.
Fission Yeast:
In fission yeast, a single Cdk namely Cdk 1(p34cdc2) and three cyclins — cig 1, cig 2 and cdc 13 are known to control cell cycle progression. Cdk1 is believed to exist in two forms — M and S forms. S form of p34cdc2– G1 cyclin (cig 1 and cig 2) complex catalyses passage through start (initiating DNA replication) and described as start promoting factor (SPF).
Alternatively M form p34cdc2 of mitotic cyclin (cdc 13) complex induces mitosis and called MPF. Thus the alteration of S phase and M phase is actually brought about by alternating activation of Cdk component of the SPF and MPF activities.
A mere association of cyclin and Cdk is not sufficient to confer kinase activity to Cdk. Besides the reversible phosphorylation of cyclin, Cdk undergoes phosphorylation and dephosphorylation. Phosphorylation of a threonine residue of Cdk subunit by Cdk activating kinase (Cak) and dephosphorylation of tyrosine residue by phosphatase results in full activity of Cdk.
Mitotic cyclin – Cdk complex initially remains inactive due to associated phosphorylation of tyrosine-15 of p34cdc2 (tyr-15 phosphorylation inhibits kinase activity). This inactive complex further undergoes phosphorylation of threonine -161 with the subsequent removal of tyrosine phosphate and Cdk 1 brings about phosphorylation of cyclin protein.
This phosphorylation and dephosphorylation bring about induction of mitosis by active MPF (Fig. 5.31).
Budding Yeast:
In budding yeast, cell cycle consists of three cycles (chromosome cycle, centrosome cycle and cytoplasmic cycle) that separate after START and join before cytokinesis.
A single Cdk, encoded by cdc28, interacts with different cyclins during different phases of cell cycle. In G1 phase, three cyclins associate with Cdk (Cdc28) — CIn1 and Cln2 phosphorylates APC to inactivate it; Cln3 have kinase activity and phosphorylates SPF and MPF to make active.
In S phase, cyclins Clb5 and Clb6 on making complex with Cdc28 induce DNA replication. Clb3 and Clb4 initiate mitotic spindle formation at the beginning of mitosis. Clb1 and Clb2 function as mitotic cyclins, on association with Cdc28, induce chromosome segregation and nuclear division (Fig. 5.32).