Study Notes on Coniferopsida. After reading this article you will learn about: 1. Introduction to Coniferopsida 2. Characteristic Features of Coniferopsida 3. Classification 4. Economic Importance 5. Systematic position of Pinus 6. Geographical distribution of Pinus 7. External Morphology 8. Internal Morphology 9. The Leaf 10. Reproduction 11. Development of Embryo and Other Details.
Introduction to Coniferopsida:
Class-Coniferopsida; Order-Coniferales; Genus-Pinus:
According to Buchholz (1948), the existing Coniferales have 50 genera and 550 species, 30 of the genera being confined to the Northern and 14 to the Southern hemisphere.
The Coniferales are well represented in the tropics but are abundant in temperate regions of the world. Many genera, e.g., Pinus, Picea, Cupressus and Juniperus are common to both Eastern and Western hemispheres. All the Pinaceae except one species of Pinus are confined to the Northern hemisphere while Araucariaceae and most of Podocarpaceae belong to the Southern hemisphere.
The Taxacae are mostly found in the Northern hemisphere. The Taxodiaceae, Cupressaceae and some of Podocarpaceae are commonly found in both the Northern and Southern hemispheres of the world. A large number of Coniferales are found abundantly in the western parts of the United States and also in the extra tropical regions of Eastern and Central Asia.
A very few conifers have been reported from tropical regions of Africa and especially of South Africa. In India, about 13 genera and 25 species of Coniferales are found.
Cephalotaxus and Taxodium have been reported to be found in South India only while the remaining 11 genera are found throughout the country especially in cool hilly places. About 7 conifers have been reported from the vicinity of Shimla, e.g., Cupressus, Pinus, Picea, Abies, Juniperus, Podocarpus and Taxus.
In Shimla hills, there are five well marked species of conifers. Pinus roxburghii (Syn. P. longifolia) is found upto a height of 6,000′ and this species is also commonly found in the plains. P. wallichiana, (Syn. V.excelsa) is found between 5,000′ to 7,000′ elevation. Cedrus deodora, the well known deodar tree is found at 8,000′ elevation or so.
Picea morindoides is found between 7,000′ to 9000′ in Shimla hills. Abies pindrow, Taxus sp. and Cupressus sp. are found at about 8,000′ elevation in Shimla hills.
About all the conifers are evergreen plants with few exceptions, e.g., dwarf junipers and few others. The trees are with a long shaft which may reach to a height of 250′ or more and may attain the girth of even 50′ or so. The conifers include the biggest trees of the world, e.g., Sequoia gigantea and Taxodium mucronatum.
All the conifers are long-lived plants and some of them may live 1,000 years and few of them like Sequoia and Taxodium sp. are said to be living for last 4,000 years or more. Lobb, an English explorer discovered these trees (Sequoia and Taxodium) in 1850 on the Sierra Nevada Mountains in California.
Dr. Schulman at the Arizona University discovered the pine trees called ‘bristle cone pines’ which he estimates to be more than 4,000 years old. They are quite small trees in their dimensions.
They are 4.5 metres to 9 metres high, 0.6 metre to 1.5 metres in diameter. These tress are now being considered the oldest living beings in the world. The species is known as Pinus aristata Englem. In our country the deodar tree attains an age of 750-900 years.
Characteristic Features of Coniferopsida:
1. Mostly evergreen with branched stems, rarely shrubs.
2. The leaves are needle or scale-like, sometimes flattened, rarely falling in autumn, spirally arranged or whorled, entire. The leaves possess xerophytic characters.
3. Wood without vessels consisting of long tracheids which show bordered pits. Resin canals present frequently. 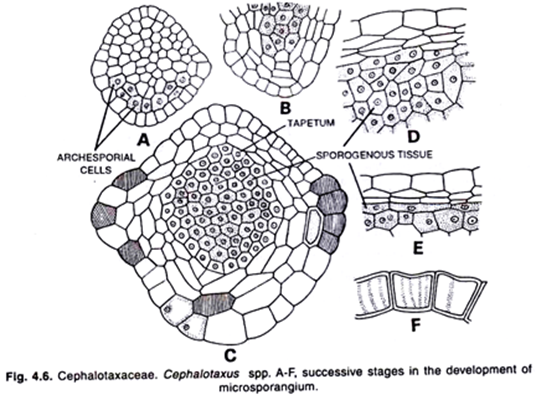
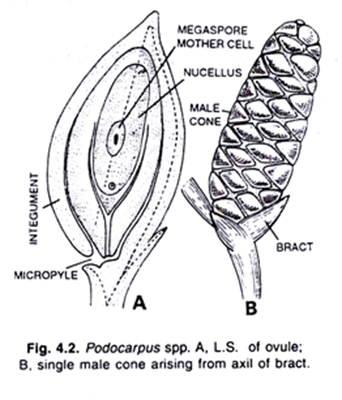
4. The flowers are monoecious or dioecious, e.g., Juniperus, Podocarpus, Taxaceae, etc. The female flowers are terminal or lateral and then surrounded by supporting bracts.
5. The male flowers consist of a number of stamens arranged in strobili. The stamens are usually many, each with 2 to 20 pollen sacs, connective often produced as an appendage.
6. Pollen grains may be winged, e.g., Pinus.
7. The female flowers are arranged in cones or catkins with the exception of Taxaceae, Cephalotaxaceae and Podocarpaceae.
8. Each female flower consists of a bract (sterile) and a scale (fertile). The scale is found above the bract. The ovules develop on the upper surface of ovuliferous scales. 
9. The female cone ripens in 1-3 years and is usually dry on ripening.
10. The seeds are often winged, nut like and with a leathery or woody testa.
11. The cotyledons are epigeal and 2-16 in number.
12. Polyembryony is quite common.
13. They produce non-motile sperms at the time of fertilization.
Classification of Coniferopsida:
The Coniferales may be divided into two main series-the Pinades and the Taxades characterized by the presence or absence of an ovulate cone. The classification of conifers depends on the worker’s personal stand point.
In fact all external features of the various parts, the superficial characters of reproductive structures and the anatomy of the vegetative and reproductive parts are taken into consideration for the study of their taxonomy.
According to Sexton, the structure of male gametophyte, the position of archegonia and the structure and development of the embryo are sufficiently distinctive to be used in the classification of conifers. He, however, recognizes only five families-1. Pinacease; 2. Cupressaceae; 3. Araucariaceae; 4. Podocarpaceae and 5. Taxaceae. He does not recognize Taxodiaceae.
Upto 1917 Chamberlain divided the conifers into two main series co-ordinate with each other and to other groups of gymnosperms-Pinaceae and Taxaceae. Chamberlain in 1935 slightly modified his view and divided the conifers into six families.
1. Abietaceae; 2. Taxodiaceae; 3. Cupressaceae; 4. Araucariacease; 5. Podocarpaceae and 6. Taxaceae. He included the first four families in his series Pinades and the last two families in series Taxades.
 Pulle has proposed a revised classification of the group.
Pulle has proposed a revised classification of the group.
It is as follows:
1. Order-Araucariales
Family-Araucariaceae
2. Order-Podocarpales
Family- Podocarpaceae
3. Order-Pinales
Family-Pinaceae
4. Order-Cupressales
Families-Cupressaceae
Taxodiaceae
5. Order-Taxales
Families-Cephalotaxaceae
Taxaceae
Economic Importance of Coniferopsida:
From the point of their utility, the conifers are the most important.
Timber:
Their timber is of universal importance. The timber is recognized for its durability, strength, lightness, elasticity, fineness in grain, etc. The conifers are abundantly found, and therefore the timber produced by them is the cheapest and the best.
Resin and turpentine:
Resin and turpentine are obtained from the coniferous trees on commercial scale. A well known resin Canada balsam is obtained from Abies balsamia. It is used as mounting medium for preparing permanent biological slides. They are a source of immense revenue to the Government of India.
Railway sleepers:
Deodar wood is the strongest of the Indian conifers. Its primary use is for railway sleepers. The average life of the sleepers being 15 years.
Newsprint paper:
A large quantity of newsprint and other rough paper is obtained from the wood of various conifers, e.g., Abies, Picea, Cryptomeria, etc.
Dry fruits:
The seeds of Pinus gerardiana are called chilgoza which are roasted and eaten.
Systematic position of Pinus:
Division. Gymnospermae
Class. Coniferopsida
Order. Coniferales
Family. Pinaceae
Genus. Pinus.
Geographical distribution of Pinus:
The genus Pinus is widely distributed in the Northern hemisphere. There are about 75 species of this genus. About six species have been recorded from different parts of our country. The blue pine, Pinus wallichiana (Syn. P. excelsa) is largely found in North-West Himalayan region at 1,800 metres to 7,000 metres elevation.
The chir pine P. roxburghii (Syn. P. longifolia) occurs from Afghanistan to Bhutan in the outer range of the Himalayas. This species is also commonly found in the Indian plains. The chilgoza pine, P. gerardiana is found in the inner arid valley of the Himalayas at 1,800 m. to 3,000 m. elevation. The Khasi pine, P. insularis is distributed in the Khasi, Naga hills and Manipur region at elevations of 750 m. to 1,950 m.
Recently a Chinese species P. armandi has also been recorded from the north-east hills of Assam. The merkus pine, P. merkusii is found in the hills of Myanmar at 150 m to 172 m elevations. In addition to these species several exotic pines have been introduced in India e.g., P. montana, P. laricio, P. sylvestris, etc.
External Morphology of Pinus:
The full-grown plant of Pinus is a large tree giving rise to a series of widespread branches. In most of pine trees a whorl of branches is produced each year. Sometimes, in young and vigorous trees two such whorls may be produced in one year. The whorls are formed in the axils of scale leaves every year.
The main shaft is cylindrical and covered with a rough scaly bark. The branching is confined to the upper part of the stem, giving a pyramid-like appearance to the plant. The branches are dimorphic, the two forms being characterized as long shoots and dwarf shoots (spurs). These shoots are also known as the shoots of unlimited growth and shoots of limited growth respectively.
There are two kinds of leaves, the scale leaves and the green acicular foliage leaves which are commonly termed as needles. The dwarf shoots or the shoots of limited growth bear the foliage leaves while the long shoots or the shoots of unlimited growth bear scale leaves on them.
The dwarf shoots with their cluster of green leaves are known as ‘spurs’. The number of needles in each spur varies from species to species. Each spur of P. monophylla is unifoliate; the spurs of P. sylvestris and P.pinaster are bifoliate; the spurs of P. roxburghii, P. gerardiqna and P. insularis are trifoliate while the spurs of P. wallichiana are pentafoliate.
The pine trees possess tap root. The tap root is elongated and possesses strong lateral roots.
The flowers are monoecious, i.e., male and female strobili (cones) are borne on the same plant. The male flowers are catkin-like (cones) but erect in position. They are found in the axils of membranous bracts which are spirally arranged on the axis.
The young female cones are solitary, paired or whorled at the apex of the current year’s shoot and consist of a central woody axis on which the two sets of scales are arranged in spiral way. The female cones are usually found on the shoots which do not bear male cones and take the place of shoots of unlimited growth.
Internal Morphology (anatomy) of Pinus:
Root:
The internal structure of root resembles to that of a dicotyledonous root. In transverse section the root shows a piliferous layer bearing unicellular root hairs. The root hairs are found only in the young roots and root tips. In young roots there is fungal growth of ectophytic mycorrhiza. With the appearance of this fungus the root hairs of the root disappear.
Just beneath the piliferous layer there lies a broad cortex which consists of 4 to 5 layers of thin-walled parenchymatous cells. The inner-most layer of the cortex is single- layered endodermis consisting of brown suberized cells containing tannin in them. Just below the endodermis there is multi-layered pericycle containing tannin and starch grains.
Lateral roots are developed from the second layer of the pericycle. The outermost layer of the pericycle helps in the formation of the digestive sac which enables the lateral roots to penetrate through the cortex to the outside. 
In the center of the stele there are two to six Y-shaped xylem bundles alternating with them. The xylem has no true vessels and consists of tracheids. The phloem consists of sieve tubes and phloem parenchyma.
Companion cells are altogether absent. In between the arms of a Y-shaped xylem bundle there lies a resin canal. In the center is a small pith.
The secondary growth takes place as in dicotyledonous roots. A cambial strip develops from parenchymtous cells in between the phloem and metaxylem. This cambium cuts secondary xylem towards the inner side and the secondary phloem towards the outside. Simultaneously a layer of the pericycle functions as a cork cambium and cuts off a layer of cork cells towards the outside.
A thick layer of cork develops which separates the cortex from the stele and because of this barrier of cork the cortex does not get food and dies.  Stem:
Stem:
The internal structure of the stem of Pinus resembles to that of a dicotyledonous stem, though on the whole it displays the simpler structure. The young stem of Pinus shows the under mentioned structure in transverse section. The young stem is somewhat wavy in outline. It is surrounded by a single-layered cuticularized epidermis.
Just beneath the epidermis there is multi-layered hypodermis consisting of lignified sclerenchymatous cells. The hypodermis constitutes the outer region of the cortex.
Underneath the hypodermis there lies the inner cortex consisting of thin-walled parenchymatous cells containing chloroplasts and resin canals. Each resin canal is surrounded by a layer of glandular epithelial cells. The innermost layer of the cortex may be considered as endodermis but it is not at all clear. The pericycle is also inconspicuous. It is parenchymatous.
The vascular bundles are conjoint, collateral and open forming a ring in the transverse section. The primary bundles are separated from each other by narrow medullary rays. The phloem consists of sieve tubes and phloem parenchyma. The sieve tubes are elongated and possess sieve plates on the radial walls. The companion cells are altogether absent.
The xylem consists of tracheids. The protoxylem consists of annular and spiral tracheids.
Secondary Growth:
The secondary growth in the stem of Pinus takes place exactly in the same way as in dicotyledonous stems. A complete ring of vascular cambium develops. It cuts the secondary xylem towards the inner side and secondary phloem towards the outside. After the secondary growth the primary xylem may be seen outside the pith region while the primary phloem is completely obliterated.
Medullary Rays:
At many places the secondary xylem and secondary phloem zones are traversed by secondary medullary rays. These vascular rays also develop from the cambial cells to replace the original primary rays. These rays are usually uniseriate. Each ray is composed of rectangular cells with thick walls and numerous simple pits. The cells contain cytoplasm, a nucleus and many starch grains.
The upper and lower margins of the medullary rays are constituted of one or two rows of marginal ray-tracheids which run horizontally and resemble short tracheids of the xylem from which they have been derived. In the cambium and phloem zones instead of marginal ray-tracheids, large thin walled cells develop which extend upwards and downwards in between the cells of vascular tissue.
In the secondary phloem the rays consist partly of starch containing cells and partly of albuminous cells. The medullary rays vary in their size. The smallest medullary ray being only two cells high and one cell wide. Usually they are only one cell broad and less than a dozen cells in height.  The secondary wood or metaxylem consists of tracheids with typical bordered pits. The tracheids are about 4 mm, long and pointed at both ends. The bordered pits are found on the radial walls and in certain species of Pinus the bordered pits are also found on tangential walls but fewer in number. The protoxylem consists of annular and spiral tracheids.
The secondary wood or metaxylem consists of tracheids with typical bordered pits. The tracheids are about 4 mm, long and pointed at both ends. The bordered pits are found on the radial walls and in certain species of Pinus the bordered pits are also found on tangential walls but fewer in number. The protoxylem consists of annular and spiral tracheids.
The resin canals are present both in primary and secondary wood. Each resin canal is surrounded by the epithelial cells. The secondary phloem consists of sieve tubes and phloem parenchyma. The sieve tubes are elongated and possess the sieve plates on the radial walls. The companion cells are altogether absent.
As seen in transverse section the secondary growth is not uniform throughout. It shows annual rings. Each annual ring represents the growth for one year. The cambium ceases its activity in the winter season which is renewed in the following spring season.
The wood formed in the spring differs from that formed in autumn. In autumn there is less supply of food material and therefore, the tracheids are formed. By counting the number of annual rings one can estimate the age of the tree. The walls of autumn tracheids are much thicker than those of spring tracheids. In the Pinus stem the wood is dense and massive and known as pycnoxylic wood.
The cork cambium or phellogen (meristematic layer) originates in the outer cortical region near the surface. This meristematic layer cuts off additional cortical cells (phelloderm) towards the inner side and forming cork towards the outside. This cork constitutes the bark which is impervious to water and protects the stem and other delicate tissues from excessive evaporation and injury. 
 In Pinus the resin cannals are found in the cortical region opposite each primary vascular bundle The resin canals form an interconnected system throughout the xylem, phloem and the cortex Each canal is bounded by a layer of glandular secreting epithelial cells, which secrete turpentine The turpentine acts as an antiseptic in healing the wounds caused by fungi or bacteria.
In Pinus the resin cannals are found in the cortical region opposite each primary vascular bundle The resin canals form an interconnected system throughout the xylem, phloem and the cortex Each canal is bounded by a layer of glandular secreting epithelial cells, which secrete turpentine The turpentine acts as an antiseptic in healing the wounds caused by fungi or bacteria.
The turpentine is oxidized to solid resin when exposed to air. This solid resin covers the wound until new bark is replaced. Sometimes, the epithelial cells become large and known as tyloses which block the resin canals.
The Leaf of Pinus:
The leaf of Pinus is xeromorphic. The whole anatomy of the leaf makes it adaptable to withstand the low temperature and scarcity of water supply.
The outline of the needle (foliage leaf) in a transverse section depends on the number of the needles in the dwarf shoot (spur). In P. monophylla the spur bears a single needle and, therefore the outline of the needle is circular. In P. sylvestris each spur consists of two needles and the outline of each needle is semi-circular.
In Pinus roxburghii and P. wallichiana each spur consists of three and five needles respectively and, therefore, the two flat faces of each needle are towards the inner side and the curved face towards the outside. In these species the outline of the needle is somewhat triangular.  The outermost layer is the epidermis which consists of extremely thick-walled and cuticularized cells. A number of depressions are found over the epidermis. The stomata are developed all over the epidermis in these depressions.
The outermost layer is the epidermis which consists of extremely thick-walled and cuticularized cells. A number of depressions are found over the epidermis. The stomata are developed all over the epidermis in these depressions.
The guard cells are sunken in depressions below the level of the epidermis. Just beneath the epidermis there is a hypodermis which is composed of one or two layers of sclerenchymatous cells. The hypodermis is several layered at the corners.
The hypodermis is interrupted by air-spaces beneath each stoma. The parenchymatous mesophyll is not differentiated into palisade and spongy tissues. It consists of thin-walled cells containing a large number of chloroplasts and starch grains.
These thin-walled cells have peg like infolding’s of cellulose projecting into their cavities. The presence of these infolding’s is probably connected with the development of the air spaces in the leaf. Beneath the hypodermis there are a number of resin passages in the mesophyll tissue. The structure of resin canal is the same as in that of the stem.  In the center of leaf there is a conspicuous endodermis encircling a many-layered pericycle. Within the pericycle there are two vascular bundles. The xylem of the bundle is being directed to the inner side and phloem towards outside. The two bundles run parallel and un-branched from base to apex of the leaf. These two vascular bundles arise from a single leaf trace.
In the center of leaf there is a conspicuous endodermis encircling a many-layered pericycle. Within the pericycle there are two vascular bundles. The xylem of the bundle is being directed to the inner side and phloem towards outside. The two bundles run parallel and un-branched from base to apex of the leaf. These two vascular bundles arise from a single leaf trace.
The pericycle consists of different types of cells. Firstly, they are parenchymatous cells rich in proteins and known as albuminous cells.
These cells are found above the phloem of vascular bundles.
The remaining tissue within the sheath is the ‘transfusion tissue’ which consists of two kinds of parechymatous cells:
1. The cells without protoplasm and pitted. 
They are tracheidal cells which serve to conduct water from the xylem to the mesophyll and they are thought to represent in function an extension of the tracheidal system. According to Worsdell, these cells have been derived from the centripetal xylem of the ancestral mesarch bundle.
2. The cells with protoplasm are not pitted. These cells are intermediate in between the mesophyll and the phloem in the transfer of food. In addition to transfusion tissue, many fibres are also found in the pericycle near the bundles.
On the whole the leaf shows many xerophytic characteristics. The leaves are acicular in form. In the transverse section the leaf shows thick cuticle, the sunken stomata, sclerenchymatous hypodermis, the simple vascular system and the peculiar transfusion tissue.
Reproduction of Pinus:
The reproduction takes place by means of spores developed in sporangia situated on the sporophylls. The strobili or cones of Pinus are monosporangiate, i.e., the two kinds (male and female cones) occur on the same plant. The plants are monoecious.
In some of the species bisporangiate strobili are occasionally produced, they have been reported in P. roxburghii by Rao (1931), in P. montana by Stell (1918) and in P. maritima by Goebel.
Male Cones:
The male cones of P .wallichiana reach maturity and shedding of pollen takes place earliest at low elevations and in hot dry weather.
The male cone is produced in the axil of a scale leaf at the base of the current year’s young shoot and thus replaces a shoot of limited growth. The cones of P. wallichiana are about 7 mm. to 1 cm. long immediately before their ripening. They lengthen to 1-2 cm. and fall soon after ripening. At the base of the cone there is an involucre consisting of a number of small imbricate scales.
The cone bears 60 to 100 spirally arranged specialized leaves known as microsporophyll’s. Each microsporophyll bears two microsporangia or pollen sacs on its underside. The development of the microsporangium is of eusporangiate type. The sporogial wall consists of four layers.
It takes place either by a single cell or by a layer of hypodermal cells. Konar and Ramchandani (1959) reported that in P. wallichiana the wall of microsporangium consists of the epidermis, two middle layers and a glandular tapetum.
Within young sporangium there is a peripheral tapetum and a central archesporial tissue which forms a number of microspore mother cells. The microspore mother cells represent the last stage of sporophyte generation. Each microspore mother cell divides meiotically producing four haploid (n) microspores.
The microspores or pollen grains separate from each other and absorb their nourishment from the disintegrating tapetal cells. According to Sethi (1928), in P. roxburghii the meiosis takes place during the last week of January.
The Microspore:
The microspore is surrounded by a three layered wall. The exine is heavily cuticularized and is found only on one side of the microspore. It does not completely cover the mature spore. The exointine or the middle layer covers the rest of the spore.
The exointine is projected outward into two large balloon-like air sacs or wings which make the spore much more buoyant and aid in its dispersal by wind. The intine or inner layer of the spore is very thin. 
On the maturation the spores germinate in situ, i.e., within the microsporagium. Firstly, the nucleus of microspore divides into two and simultaneously a wall develops in between these two daughter nuclei forming a very small flattened prothallial and a large tube cell.
At this stage the microsporangia burst by longitudinal slits and the microspores are shed. The shedding of the pollen grains of P. wallichiana takes place in hot dry weather from the end of April to the beginning of June in our country.
The Female Cone:
The female cones develop laterally in the axil of scale leaves. They are formed in clusters in place of long shoots, i.e., shoots of unlimited growth. They are produced on the different branches from those of the male cones. There may be one to four cones on each long shoot.
According to Sethi, the female cones are initiated in January and become visible in February at the ends of youngest female shoots. In P. wallichiana the young female cones are pollinated from the end of April to the beginning of June.
The young female cones of P.wallichiana, at the time of pollination are 10-13 mm. long and 3.8 mm. in diameter. The young cones are dark reddish purple in colour. Each cone consists of central axis bearing spirally arranged scales. At the base of the cone certain sterile scales are also found.
Each fertile scale consists of two structures. 1. bract scales or cover scales which are arranged spirally and developed directly from the cone axis, and 2. The ovuliferous scale which develops on the upper surface of the bract scale.
The bract scale is leathery while the ovuliferous scale is woody in structure. Each ovuliferous scale bears two ovules on its upper surface. The bract scales are concealed under the ovuliferous scales and, therefore, they are not visible from outside.
The ovulate strobilus of Pinaceae has long been a morphological puzzle. The question arises whether structure of ovulate strobilus is simple or compound. There is much confusion about the double structure of the strobilus.
The views of the various workers are as follows:
Robert Brown regarded the ovuliferous scale as an open carpel. Alexander Braun (1853) stated that the ovuliferous scale represents the first two leaves of an axillary shoot which are fused by their upper margins. This view was accepted by many botanists of the time.
According to Sachs and Eichler, the ovuliferous scale is an outgrowth of the bract scale comparable to a ligule or placenta. Kubart and Bessey regarded the ovuliferous scale as a combined outgrowth of the ovules themselves and called an aril or an enlargement of the chalaza of the ovules.
According to Delpino the ovuliferous scale develops from two lateral lobes of the bract scale which have been turned inwards and fused together. Hirmer states that the ovuliferous scale and the bract scale are both parts of one structure which has forked vertically in the Cheirostrobus. Florin, however, regards the cone as a compound structure. He regards the cone as inflorescence and the ovuliferous scales as short shoots.
The Ovule:
The two ovules are found side by side on the upper surface of the ovuliferous scale. Each ovule consists of a group of the cells forming a tissue, the nucellus. The ovule is surrounded by a two-lipped covering known as integument. The integument develops up around the ovule.
It starts from the abaxial or outer end of the nucellus and grows inwards towards the base of the ovuliferous scale. The integument completely surrounds the nucellus except at the inner and where a wide aperture lies, known as micropyle.
The integument is fused to the nucellus except for a short distance near the micropyle. At the apex of the young nucellus a single deep seated hypodermal cell is found. It is known as archesporial cell. The archesporial cell divides forming a tapetal cell and a megaspore mother cell.
The megaspore mother cell divides meiotically producing a linear tetrad of four haploid megaspores. The three upper megaspores of the linear tetrad generate leaving the lowermost functional megaspore of embryosac.
As regards the development the sporogeneous tissue is smal and hypodermal. The hypodermal cells cut off the tapetum which forms a more or less nutritive layer around the megaspore. This is described as spongy tissue, which is formed by tapetum surrounding the megaspore mother cell. 
At this stage the pollination takes place. The development of the embryo-sac takes place after pollination.
Pollination:
In Pinus the pollination is effected by the wind. The balloon-like wings of the pollen grains aid them to reach the ovule. Usually the pollination occurs towards the end of May when the pollen grains are liberated in large quantities from the microsporangia of male flowers. The pollen grains fall on the scales of the female cone.
At this time, the edges of the bract scales become incurved separating the ovuliferous scales, which allow the pollen grains to reach to the ovules. Prior to the pollination the scales remain tightly closed around the cone axis and soon after pollination the scales close again and remain in the same condition until the ripening of the seeds.
At the time of the pollination the yellow clouds of the microspores may be seen in the atmosphere. Most of the pollen grains go waste. As the ovuliferous scales are separated from each other, the nucellus secretes a mucilaginous fluid. The pollen grains become entangled in this mucilaginous fluid.
As the pollination is over, this drop of mucilaginous fluid dries up, drawing the pollen grains through the micropyle to the apex of the nucellus, where they germinate. After pollination the scales close, the young cones increase somewhat in size and begin to become hard. 
The Male Gametophyte and its Development:
At the time of pollination the first nuclear division has already occurred in the pollen grain. The first prothallial cell and the tube cell have also been formed at this stage. The further development of the male gametophyte takes place when it germinates on the nucellus.
The tube cell divides forming a small second prothallial cell and a large tube cell. The first and second prothallial cells soon become flattened and disintegrate. The remaining nucleus divides third time producing a large rounded cell, the antheridial cell on top of the degenerating prothallial cells. The nucleus which remains in the tube cell of the pollen grain is known as tube nucleus. 
On making the contact with the apex of the nucleus the exointine of the microspore or pollen grain breaks between the wings, and the inline grows out in the form of the pollen tube. The tube nucleus passes in the pollen tube. The pollen tube penetrates the nucellar tissue and grows slowly throughout the following summer.
Now the scales of the female cone become thickened and thus the female cone becomes completely closed. The developing pollen rests there throughout the winter. In the next April, the antheridial cell divides into two cells, forming the body cell and stalk cell. The stalk cell does not divide further.
The body cell divides into two unequal cells with less cytoplasm and large nuclei. These cells are known as male cells (see fig. 4.42).
The Female Gametophyte and its Development:
During the period between pollination and fertilization many changes take place in the ovule and the cone as a whole. The female cone increases in size and becomes green. This increase in size of the cone takes place due to the enormous growth of the axis and of the ovuliferous scales.
The megaspore is formed when the surrounding nucellus is in earlier stages of development. Both the megaspore and nucellus continue to grow simultaneously. The nucleus within the megaspore enlarges and begins to divide. Free nuclear division takes place and from 256 to more than two thousand nuclei are formed within the germinating megaspore.
After free nuclear division a large vacuole appears in the centre and all nuclei now come to lie near periphery. After this peripheral disposition of the nuclei the wall formation starts. During all this time the megaspore gradually increases in size and its membrane that of the ovule becomes thicker. 
The wall formation takes place by the formation of perpendicular walls from the periphery and gradually proceeds towards the centre. As the cell formation proceeds towards the centre, in the beginning the cells remain open, and towards the inner side in the megaspore the walls are laid down.
In P. wallichiana, during the first year the functional megaspore undergoes only a few free nuclear divisions, and in the next year the free nuclear division continues and cell formation begins in the following May.
Thereafter the embryo sac or megaspore is cut into a number of radial spaces in the form of long tubes, containing several nuclei described as alveoli. These alveoli project towards the centre of the germinating megaspore. They are open on the inner side in the beginning but later on they become closed and new cross walls are laid down in these tubes.
They form the endosperm or female prothallus (female gametophyte) within the megaspore.
In early stages of the development of megaspore, it is surrounded by a layer of cells formed by tapetum, this layer forms the endosperm jacket. The nutritive matter is gradually passed on from the surrounding nucellus to the developing endosperm (female prothallus). The nucellus gradually decreases in size.
In Pinus the formation of archegonia starts at an early stage of the formation of endosperm or prothallus (female gametophyte). In Pinus the archegonia are quite separate from each other. At the micropylar end of the ovule the archegonia are produced from superficial cells of the female prothallus.
In many species of Pinus the number of archegonia ranges from one to five, but usually two or three achegonia is found. Each archegonium is quite simple in structure and consists of a short neck and a large venter.
As regards the development of the archegonium the archegonial initial divides producing two cells- the primary neck cell and the central cell. The primary neck cell divides thrice successively producing eight cells which constitute the neck. Usually the neck consists of eight cells arranged in two tiers of four cells each.
The central cell divides to produce an oosphere (egg) and the ventral canal cell. The cell surrounding the archegonium form a jacket layer which supplies food to the developing egg. The cells of the female prothallus surrounding the archegonium grow more vigorously than the neck cells so that an archegonial chamber is resulted.
Fertilization:
In the month of April of the second year the pollen tube completes its resting period and again becomes active and the two unequal male cells are formed from the body cell. The pollen tube elongates until it reaches the neck of an archegonium. The end of the pollen tube enters the neck and bursts discharging the stalk cell, tube nucleus and the two unequal male cells.
The larger nucleus enters the egg and fuses with the female nucleus, thus effecting the fertilization. The other nuclei, however, die and disappear. With the result of fertilization, a thick-walled, diploid (2n) oospore is formed. The fertilization is completed by the end of June.
Development of Embryo of Pinus:
In Pinus, the free nuclear division of the fertilized egg takes place in the early embryogeny and the number of nuclei formed is very low as compared to that of Cycas. The oospore is also smaller than that of Cycas.
In Pinus, the fertilized diploid nucleus of the oospore divides twice to form four nuclei which come to lie side by side at the bottom of the egg. Thereafter a third nuclear division takes place forming the eight nuclei which are arranged in two tiers of four each.
The cell walls are laid down across the four basal nuclei, and this way four small cells have been produced at the lower end of the oospore. The upper free nuclei are separated only by imperfect walls and they do not take any part in the formation of the embryo. The lower cells again divide and this way, three tiers of four cells each have been formed.
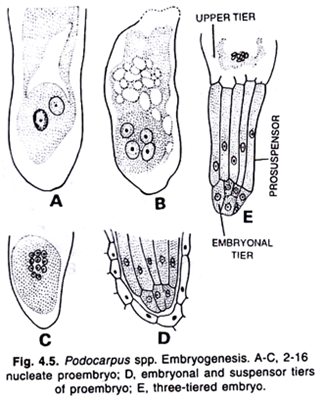
Once again the lowermost nuclei divide and four tiers of 4 cells each have been formed. This structure consists of sixteen cells, four tiers of four cells each, is known as proembryo.
The cells of the uppermost tier remain open towards the cavity of egg. This tier of four cells is known as open tier. The second tier of four cells constitutes the rosette tier. The cells of this tier are functionless. The third tier consists of elongated cells is known as suspensor tier. The cells of suspensor tier form the elongated suspensors.
The fourth and the lowermost tier of the four cells is known as embryonal tier. The cells of this tier give rise to the embryo proper. It is seep in some cases (Buchholz) that rosette cells are also meristematic and give rise to secondary embryos.
The suspensors elongate very greatly and thrust down the embryonal cells into the tissue of the female prothallus.
There is a peculiar feature of embryogeny in Pinus that the four embryonal cells generally become separated from each other and give rise to four independent embryos. Each of such embryos develops secondary suspensor cells. When more than one embryo is formed from single fertilized egg is called cleavage polyembryony.
Ultimately, only one of these embryos survives and becomes mature. In Pinus, the cleavage polyembryony can be recognized both by counting the number of embryos produced from each zygote and by tracing each embryo back to single-celled suspensor (Buchholz, 1926).
Structure of Embryo:
The mature embryo consists of a short axis with the radicle towards the micropylar end and a small plumule downwards. The plumule is surrounded by a number of tiny leaves or cotyledons. The cotyledons may be ten or so in number. The suspensor remains attached as a small thin coil to the tip of the radicle which forms a thick cap over it.
The mature embryo is embedded in the endosperm which is laden with food material. The nucellar tissue is altogether crushed and disorganized because of the expansion of endosperm and embryo, however, the remaining thin layer of nucellus is known as perisperm. 
The Seed of Pinus:
The seed contains the following structure:
1. Embryo:
It contains a straight mature embryo. The structure of the mature embryo has already been described in preceding lines.
2. Endosperm:
The endosperm is laden with food material. The embryo remains embedded in the endosperm and absorbs its nutrition from it.
3. Perisperm:
A thin layer of crushed nucellus persists which is known as perisperm.
4. Testa:
The integument of the ovule becomes the seed coat or testa. The testa is hard and stony as it develops from the middle stony layer of the integument.
5. Wing:
The seed has a thin membranous wing which is derived from the surface of the ovuliferous scale. The wing helps in the dispersal of the seed.
Dispersal of Seeds:
In the third year when the female cone reaches maturity the cone becomes dry, brown and woody. Each ovuliferous scale bears two mature seeds placed side by side on its upper surface. When the seeds are mature the axis of cone elongates and the scales are separated from each other leaving spaces among them.
The seeds are liberated and they are blown away by the wind. The wings of the seeds aid in their dispersal.
Germination of Seed:
The pine seeds may germinate immediately after they fall on moist soil. The seeds may also remain dormant for some time in unfavourable conditions. In favourable conditions the seed absorbs water and the seed coat splits up. On splitting the seed coat the radicle grows downward in the soil and plumule upwards towards the light.
The green cotyledons carry up the remaining part of the seed and the tips of the cotyledons absorb the remainder of the endosperm.  The radicle goes downwards and forms the primary tap root while the plumule which passes upwards produces a shoot of unlimited growth which bears delicate acicular green leaves. These leaves are spirally arranged on the shoot. The germination of pine seed is epigeal as the cotyledons come upwards above the soil.
The radicle goes downwards and forms the primary tap root while the plumule which passes upwards produces a shoot of unlimited growth which bears delicate acicular green leaves. These leaves are spirally arranged on the shoot. The germination of pine seed is epigeal as the cotyledons come upwards above the soil.
This condition of juvenile leaves prevails until the seedling is 3 or 4 inch high and thereafter first foliar spurs (dwarf shoots) develop in the axils of these leaves. The juvenile condition is a primitive feature of Pinus. The later formed juvenile leaves of the seedling become smaller and smaller and pass on into the scale leaves of the stem.
Economic Importance of Pinus:
Several species of Pinus yield wood which is used for building material, furniture, poles, match boxes and other such articles. The wood of P. wallichiana (Kail) and P. roxburghii (Chir) yeild good timber. The wood is also used as fuel because it catches fire quite easily because of the presence of resin. The old female cones are also used as fuel and decoration pieces.
The pine trees yield large amount of resin which is used to manufacture turpentine. Resin and turpentine are used to make paints, varnishes and medicines.
The seeds of P. geradiana are edible and known as ‘chilgoza’. The seeds of P. roxburghii are also edible.
Many species of Pinus make cheap sources of cellulose which is used for various purposes. Usually the T.B. sanatoria are situated in the pine forests because of attractive and healthy atmosphere.
In India P. gerardiana provides edible seeds, ‘chilgoza’; P. roxburghii and P. wallichiana yield timber; P. roxburghii and P. insularis yield resin and turpentine.