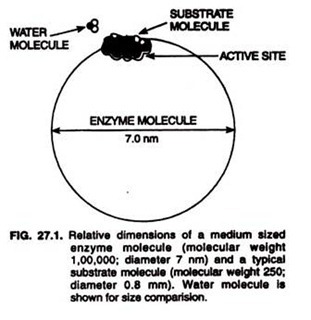
Let us make an in-depth study of the blood. After reading this article you will learn about the composition of blood: a. Blood Cells and b. Blood Plasma.
Blood:
Blood is the major fluid present in the body that flows through the blood vessels (veins, arteries & capillaries) and helps to connect, communicate, supply and drain substances to and from various parts of the body. The total volume of blood in an adult male human being is about 5 litres.
It has a specific gravity of 1.055 to 1.065. The pH of blood ranges from 7.35 to 7.45 average being 7.4, however the pH of blood varies from 7.3 to 7.5 without disturbing the normal functions of the body. The pH of intracellular fluid (cytoplasm) is 7.2.
Composition of blood:
Blood is composed of:
(a) Blood cells which include:
(i) Erythrocytes
(ii) Leukocytes and
(iii) Platelets.
(b) Blood plasma.
Blood Plasma:
It is the fluid remaining after the removal of blood cells. Plasma devoid of clotting factors is termed as serum i.e. plasma — fibrinogen = serum. Plasma contains the plasma proteins, organic constituents (glucose, amino acids, fatty acids, urea, cholesterol etc.) and inorganic constitutes (phosphates, K, Na, CI, and Fe). All these are suspended in a fluid form which constitutes about 90 % water.
Plasma proteins:
Proteins in the plasma can be detected by Biuret test. The amount of protein is estimated by conducting micro-biuret test. Upon estimation it is found that the amount of plasma proteins ranges from 6.5 to 7.5 gms/dl.
Methods to separate different types of plasma proteins:
1. Electrophoresis
2. Isoelectric focusing
3. Precipitation using salts
4. Precipitation using alcohol
5. Precipitation using heavy metals
6. Ultracentrifugation.
Electrophoresis:
Plasma, which has a pH of 7.4 is rendered alkaline and brought to a pH of 8.6 and maintained by barbitone buffer. At pH 8.6, all plasma proteins will have a negative charge i.e. they exist in anionic form. A drop of this solution is applied on to a piece of filter paper (or a dish with polyacrylamide gel) and this filter paper is dipped in a buffer (barbitone).
This strip is subjected to electrophoresis by applying electric current. When current is applied, all the proteins move towards anode (+ve). But different proteins move with different rates which depend on the intensity of charge present on them. Different proteins exhibit the various number of charges on them. Current is allowed to pass for a short time interval after which it is stopped. Paper is stained with a dye. On staining it shows a number of separate bands.
The band close to anode corresponds to albumin, other bands in the order are α1 -globulin, α2-globulin, β-globulin, fibrinogen and γ-globulin (closest to cathode). When the plasma proteins are separated on polyacrylamide gel both molecular weight and charge contribute to their separation in the same order.
Salting-out technique:
Most of the proteins are insoluble in pure water. But when some amount of salt is added to this solution then these proteins become soluble, this is known as salting-in of proteins. When high amounts of the same salt is added to the same solution then the proteins precipitate out of the solution, this is known as salting-out of proteins. When protein water interaction increases the protein becomes soluble but when protein-protein interaction increases then protein precipitates out of the solution.
In the absence of the salt there is neither protein water interaction nor protein-protein interaction, thereby the protein is neither soluble nor is precipitated. Now, when a small amount of the salt is added, it associates with the protein molecule creating an atmosphere around the protein molecule thereby increasing protein-water interaction and thus making protein soluble in the water i.e. salting-in of proteins.
When the salt is added in high amounts, the ions of the salt start associating with the water molecules thereby reducing the protein-water interaction and increasing protein-protein interactions. As the protein- protein interaction increases, precipitation increases i.e. salting-out of proteins.
Concentrated salt solutions, like sodium sulphate, sodium chloride and ammonium sulphate etc. precipitate proteins by neutralization and dehydration, as well as absorption, thereby converting emulsions into suspensoids, which results in the precipitation. Different proteins precipitate out with different salts at varying concentrations; this forms the basis of separation of proteins.
Precipitation using alcohols and heavy metals:
Using different concentrations of alcohol different proteins are precipitated. Heavy metals like zinc (Zn), lead (Pb) and mercury (Hg) are used to precipitate the plasma proteins.
Fingerprinting technique:
When polypeptide chains, obtained by enzymatic hydrolysis of a protein are subjected to two dimension paper chromatography or two dimensional paper electrophoresis or paper chromatography in one direction and paper electrophoresis in the other (i.e. 90 degrees to the first), give a particular pattern or map unique to that protein. As the pattern for each protein is unique this map is used as a fingerprint, which helps in the identification and separation of proteins and amino acids.
Fingerprinting technique enables the determination of protein sequence and differentiation of abnormal protein from normal. In order to differentiate normal hemoglobin (HbA) from abnormal sickle cell hemoglobin (HbS), both the proteins are separately digested by trypsin.
The hemoglobin digest is applied on a filter paper and subjected to electrophoresis in one direction and paper chromatography in the other direction. On treatment with ninhydrin, the map obtained for HbS differs in one position from the normal thereby indicating that one of the polypeptides has been changed. Separation and characterization of plasma proteins revealed the following proteins.
Albumin:
It is present in highest concentration than other plasma proteins. Its concentration ranges from 3.5-5.5 gms/100 ml. It has the lowest isoelectric pH of 4.7. It migrates fastest in electrophoresis and precipitates last in salting out or alcohol precipitation methods. It is a simple protein synthesized in the liver. It is of nutritional value because it contains all essential amino acids. It coagulates on heating and precipitates on full saturation with ammonium sulphate.
Functions:
(a) Stores amino acids and supplies amino acids to extra hepatic tissues.
(b) Maintains the colloidal osmotic pressure of plasma (oncotic pressure).
(c) Deficiency of albumin leads to oedema because albumin is the major protein that regulates water content of the tissues.
(d) It is a carrier protein. It transports fatty acids, steroid hormones, calcium, thyroxine, toxic substances like bilirubin, urobilinogen and phenolic substances across the blood.
(e) It acts as a buffer.
Concentration of albumin decreases in hepatic disorders, malnutrition, increased excretion of albumin as seen in kidney disorders (nephrosis). Concentration of albumin increases in dehydration and insulin resistant diabetes.
Globulin:
Its concentration is about 2-5 gms/dl. It is synthesized in the extra hepatic tissue (lymphoid). However a minor quantity is also synthesized in the hepatic tissue. The albumin/globulin ratio is 1.2 to 1.8, average being 1.5. Decreased AG ratio indicates decreased albumin. Increased AG ratio indicates decreased globulin.