In this article we will discuss about Denaturation and Renaturation of DNA Double Helix.
Denaturation of DNA:
Denaturation of DNA double helix takes place by the following denaturating agents:
(i) Denaturation by Temperature:
If a DNA solution is heated to approximately 90°C or above there will be enough kinetic energy to denature the DNA completely causing it to separate into single strands. This denaturation is very abrupt and is accelerated by chemical reagents like urea and formamide.
The chemicals enhance the aqueous solubility of the purine and pyrimidine groups. This separation of double helix is called melting as it occurs abruptly at a certain characteristic temperature called denaturation temperature or melting temperature (Tm).
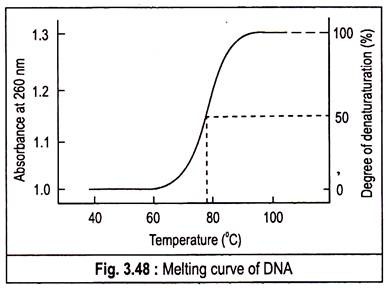
It is defined as temperature at which 50% of the DNA is melted. The abruptness of the transition indicates that the DNA double helix is highly cooperative structure, held together by many reinforcing bonds. The melting of DNA can be followed spectrophotometrically by monitoring the absorbance of DNA at 260 nm. Tm is analogous to the melting point of crystal. The Tm value depends on the nature of the DNA.
If several samples of DNA are melted, it is found that the Tm is highest for those DNAs that contain the highest proportion of G—C. Actually the value is used to estimate the percentage of G—C in a DNA sample. In fact, the Tm of DNA from many species varies linearly with G—C content.
This relationship between Tm and G—C content arises due to guanine and cytosine form three hydrogen bonds when base paired, whereas adenine and thymine form only two.
Denaturation involves the following changes of the properties of DNA:
(a) Increase in Absorption of UV-Light:
If denaturation is followed spectrophotometrically by monitoring the absorbance of light at 260 nm, it is observed that the absorbance at 260 nm increases as the DNA become denatured, a phenomenon known as the hyperchromatic effect or hyperchromacity or hyperchromism. This is due to un-stacking of base pairs.
A plot of the absorbance at 260 nm against the temperature of a DNA solution indicates that little denaturation occurs below approximately 70°C, but further increases in temperature result in a marked increase in the extent of denaturation.
(b) Decrease in Specific Optical Rotation:
Double-stranded DNA shows a strong positive rotation which highly decreases with denaturation. This change is analogous to the change in rotation observed when the proteins are denatured.
(c) Decrease in Viscosity:
The solutions of native DNA exhibit high viscosity because of the relatively rigid double helical, long and rod like character of DNA molecule. Denaturation causes a marked decrease in viscosity.
If melted DNA is cooled it is possible to reassociate the separated strands, a process known as renaturation. However, a stable double-stranded molecule may be formed only if the complementary strands collide in such a way that their bases are paired precisely. But renaturation may not be precise if the DNA is very long and complex.
Thus the rate of renaturation (renaturation kinetics) can give information about the complexity of a DNA molecule. Complete denaturation is not a readily reversible process. If a heat-denatured DNA solution is cooled slowly (anneling) and hold the solution at about 25°C below Tm and above a concentration of 0.4M Na+ for several hours, some amount of
DNA (50-60%) is renatured. Rapid cooling does not reverse denaturation, but if the cooled solution is again heated and then cooled slowly, renaturation takes place.
(ii) Denaturation by Chemical Agents:
Denaturation of DNA double helix can also be brought about by certain chemical agents such as urea and formamide. These chemical reagents enhance the aqueous solubility of the purine and pyrimidine groups. The Tm value is lowered by the addition of urea. In 8M urea, Tm is decreased by nearly 20°C. DNA can be completely denatured by 95% formamide at room temperature only.
(iii) Effect of pH on Denaturation:
Denaturation also occurs at acidic and alkaline solutions in which ionic changes of the purine and pyrimidine bases can occur. In acidic solutions at pH values 2-3 the amino groups bind with protons and the DNA double helix is disrupted. Similarly, in alkaline solutions at pH 12, the enolic hydroxyl groups ionize, thus preventing the keto-amino hydrogen bonding.
Renaturation of DNA:
When preparations of double-stranded DNA are denatured and allowed to renature, the rate of renaturation can give valuable information about the complexity of the DNA if there are repetitive sequences in the DNA, it shows less complexity in comparison to its total length, but the complexity is equal to its total length if all sequences are unique.
The 1kb DNA fragments are denatured by heating above its Tm and then renatured at a temperature 10°C below the Tm and monitored either by decrease in absorbance at 260 nm (hypochromic effect), or by passing samples at intervals through a column of hydroxylapatite, which retains only double stranded DNAs, and estimating how much of the sample is retained.
The degree of renaturation after a given time depends on C0, the concentration of double stranded DNA prior to denaturation, and t, the duration of the renaturation in seconds. The concentration is measured in nucleotides per unit volume. In order to compare the rates of renaturation of different samples of DNA it is usual to measure C0 and the time taken for renaturation to proceed half way to completion, t1/2, and to multiply these values together to give a C0t1/2 value. The larger the C0t1/2, the greater the complexity of the DNA; hence λ DNA has a far lower C0t1/2 than does human DNA.
if the extent of renaturation is plotted against log C0t (known as Cot curve), it is observed that part of the DNA is renatured quite rapidly while the rest is very slow to renature. This indicates that some sequences have a higher concentration than others i.e., part of the genome consists of repetitive sequences.
These repetitive sequences can be separated from the single-copy unique DNA by passing the renaturating sample through a hydroxylapatite column early in the renaturation process, at a time which gives a low value of C0t. At this stage only the rapidly renaturating sequences will be double stranded, and will, therefore, bind to the column.