Some of the important types of Spectroscopic Techniques are as follows:
Contents
- Type # 1. Gamma Spectroscopy:
- Type # 2. X-ray Spectroscopy:
- Type # 3. Ultraviolet-Visible Spectroscopy:
- Type # 4. Fluorescence Spectroscopy:
- Type # 5. Circular Dichroism Spectroscopy:
- Type # 6. Atomic Spectroscopy:
- Type # 7. Infrared Spectroscopy:
- Type # 8. Raman Spectroscopy:
- Type # 9. Mass Spectroscopy:
- Type # 10. Electron Paramagnetic Resonance:
- Type # 11. NMR Spectroscopy:
- Type # 12. Laser-Induced Breakdown Spectroscopy:
- Type # 13. Mossbauer Spectroscopy:
- Type # 14. Rotational Spectroscopy:
Type # 1. Gamma Spectroscopy:
Gamma spectroscopy is a radionuclide measurement method. While a Geiger counter determines only the count rate, a gamma spectrometer will determine the energy and the count rate of gamma-rays emitted by radioactive substances.
Gamma spectroscopy is an extremely important method. Most radioactive sources produce gamma-rays of various energies and intensities.
When these emissions are collected and analyzed with a gamma spectroscopy system, a gamma energy spectrum can be produced. A detailed analysis of this spectrum is typically used to determine the identity and quantity of gamma emitters present in the source. The gamma spectrum is characteristic of the gamma emitting nuclides contained in the source, just as in optical spectroscopy, the optical spectrum is characteristic of the atoms and molecules contained in the probe.
The equipment used in gamma spectroscopy includes an energy sensitive radiation detector, a pulse sorter (multichannel analyzer), and associated amplifiers and data readout devices. The most common detectors include sodium iodide (Nal) scintillation counter and high purity germanium detectors.
Instrumentation:
A gamma spectroscopy system consists of a detector, electronic system to collect and process the signals produced by the detector, and a computer with processing software to generate the spectrum, and display and store it for analysis. Gamma spectroscopy detectors are passive materials that wait for a gamma interaction to occur in the detector volume.
The most important interaction mechanisms are the photoelectric effect, the Compton Effect, or pair production. The photoelectric effect is preferred, as it absorbs all of the energy of the incident gamma-ray. Full energy absorption is also possible when a series of these interaction mechanisms take place within the detector volume.
When a gamma-ray undergoes a Compton interaction or Pair Production, and a portion of the energy escapes from the detector volume without being absorbed, the background rate in the spectrum is increased by one count. This count will appear in a channel below the channel that corresponds to the full energy of the gamma-ray. Larger detector volumes reduce this effect.
The voltage pulse produced by the detector (or by the photomultiplier in a scintillation detector) is shaped by a multichannel analyzer (MCA). The multichannel analyzer takes the very small voltage signal produced by the detector, reshapes it into a Gaussian or trapezoidal shape, and converts it into a digital signal. In some systems, the analog to digital conversion is performed before the peak is reshaped.
The analog to digital converter (ADC) also sorts the pulses by their height. ADCs have specific numbers of “bins” to sort the pulses into; these are the “channels” in the spectrum. The number of channels can be changed in most modern gamma spectroscopy system by changing a software or hardware setting.
The number of bins is a power of two. Common values include 512, 1024, 2048, 4096, 8192, or 16384 channels. The choice of number of channels depends on the resolution of the system and the energy range being studied. The MCA output is sent to a computer which stores, displays, and analyzes the data.
A variety of software packages are available from several manufacturers, and generally include spectrum analysis tools such as energy calibration, peak area and net area calculation, and resolution calculation. Other components, such as rate meters and peak position stabilizers, may also be included. Gamma spectroscopy systems are selected to take advantage of several performance characteristics. Two of the most important include detector resolution and detector efficiency.
1. Detector Resolution:
Gamma rays detected in a spectroscopic system produce peaks in the spectrum. These peaks can also be called lines, by analogy to optical spectroscopy. The width of the peaks is determined by the resolution of the detector, a very important characteristic of gamma spectroscopic detectors. Resolution is analogous to Resolving power in optical spectroscopy.
High resolution enables the spectroscopist to separate two gamma lines which are close to each other. Gamma spectroscopy systems are designed and adjusted to produce symmetrical peaks of the best possible resolution. The peak shape is usually a Gaussian distribution. In most spectra, the horizontal position of the peak is determined by the gamma-ray’s energy, and the area of the peak is determined by the intensity of the gamma-ray and the efficiency of the detector.
The most common figure used to express detector resolution is Full Width at Half Maximum (FWHM). This is the width of the gamma-ray peak at half of the highest point on the peak distribution. Resolution figures should be given with reference to specified gamma-ray energies. Resolution can be expressed in absolute terms (eV or keV), or relative terms.
For example, a Nal detector may have a FWHM of 9.15 keV at 122 keV, and 82.75 keV at 662 keV. These are resolution values expressed in absolute terms. To express the resolution in relative terms, the FWHM in eV or keV are divided by the energy of the gamma-ray and multiplied by 100. In these examples, the resolution is 7.5% at 122 keV, and 12.5% at 662 keV. A Germanium detector might give resolution of 560 eV at 122 keV, a relative resolution of 0.46%.
2. Detector Efficiency:
Not all gamma-rays that are emitted by the source and pass through the detector will produce a count in the system. The probability that an emitted gamma-ray will interact with the detector and produce a count is the efficiency of the detector. High efficiency detectors produce spectra in less time than low efficiency detectors.
In general, larger detectors have higher efficiency than smaller detectors, although the shielding properties of the detector material are also an important factor. Detector efficiency is measured by taking a spectrum from a source of known activity, and comparing the count rates in each peak to the count rates expected from the known intensities of each gamma-ray.
Efficiency, like resolution, can also be expressed in absolute or relative terms. The same units are used, percentages, so the spectroscopist must take care to determine which kind of efficiency is being given for the detector. Absolute efficiency values give the probability that a gamma ray of a specified energy passing through the detector will interact with the crystal and be detected.
Relative efficiency values are often used for Germanium detectors, and compare the efficiency of the detector at 1332 keV to that of a 3″ by 3″ Nal detector (1.2E-3 cps/Bq). Relative efficiency values greater than 100% can, therefore, be encountered when working with very large Germanium detectors.
The energy of the gamma-rays being detected is an important factor in the efficiency of the detector. By plotting the efficiency at various energies, an efficiency curve can be obtained. This curve can then be used to determine the efficiency of the detector at energies different from those used to obtain the curve.
Scintillation Detectors:
Scintillation detectors use crystals that emit light when gamma-rays interact with the atoms in the crystals. The intensity of the light produced is proportional to the energy deposited in the crystal by the gamma-ray. The mechanism is similar to that in a Thermo-luminescent Dosimeter. The detectors are joined to photomultipliers that convert the light into electrons and amplify the electrical signal provided by the electrons.
Because the photomultipliers are also sensitive to ambient light, scintillators are packaged in light-tight coverings. Common scintillators include Thallium-doped Sodium Iodide (NaI(Tl)), often simply called Sodium Iodide (Nal) detectors, and Bismuth germinate (BG) detectors. Scintillation detectors also have other uses, such as alpha- and beta-detectors.
(a) Sodium Iodide {Nal(Tl)} Detectors:
Thallium-doped Sodium iodide has two principal advantages; it can be produced in large crystals, giving good efficiency, and it produces intense bursts of light compared to other spectroscopic scintillators. It is also convenient to use, making it popular for field work such as identification of unknown materials for law enforcement purposes.
An example of a Nal spectrum is the gamma spectrum of the isotope 137Cs shown in the illustration. 137Cs emits a single gamma line of 662 keV. It should be noted that the 662 keV line shown is actually produced by 137Bam, the decay product of 137Cs, which is in secular equilibrium with 137Cs.
The spectrum was measured using a Nal-crystal on a photomultiplier, an amplifier and a multichannel analyzer and plotted on an x plotter. The figure shows the number of counts (within the measuring period) versus channel number. The spectrum shows the following peaks (from left to right):
i. Low energy x radiation (due to internal conversion of the gamma-ray)
ii. A backscatter peak at the low energy end of the Compton distribution
iii. A photo peak (Full Energy Peak) at an energy of 662 keV
The Compton distribution is a continuous distribution which goes up to channel 150 in this figure. It is due to primary gamma-rays undergoing Compton Effect within the crystal. Depending on the scattering angle, the Compton electrons have different energies and hence produce pulses of different heights. If many gamma-rays are present in a spectrum, Compton distributions are a disturbing nuisance. In order to reduce them, one can use an anticoincidence shield. This is especially useful for small Ge(Li) detectors.
The next figure shows another example: the gamma spectrum of the isotope 60Co with two gamma-rays with 1.17 MeV and 1.33 MeV, respectively, again measured by a Nal counter. The two gamma lines can be seen well separated; the rise to the left of channel 200 probably indicates a strong background that has not been subtracted.
At channel 150, one can see a backscatter peak (like in the figure above). The multichannel spectrum was plotted by means of an x-y plotter. Sodium Iodide systems, like all scintillator systems, are sensitive to temperature changes. Changes in the operating temperature caused by changes in environmental temperature will shift the spectrum on the horizontal axis.
Peak shifts of tens of channels or more are commonly observed. Spectrum stabilizers art used to prevent such shifts. Because of their poor resolution, Nal detectors are not suitable for the identification of complicated mixtures of gamma-ray producing materials. Such analyses require higher resolution.
Semiconductor Detectors:
Semiconductor detectors are also called solid-state detectors. These detectors are fundamentally different from Scintillation detectors. In semiconductor detectors, an electric field is applied to the detector volume. An electron in the semiconductor is fixed in its Valence band in the crystal until a gamma-ray interaction gives the electron enough energy to move to the Conduction band.
Electrons in the conduction band can respond to the electric field in the detector, and therefore, move to the positive contact that is creating the electrical field. The gap where the electron used to be is called a “hole” and is filled by an adjacent electron. This shuffling of holes effectively moves a positive charge to the negative contact.
The arrival of the electron at the positive contact and the hole at the negative contact produces the electrical signal that is sent to the preamplifier, the MCA and on through the system for analysis. The movement of electrons and holes in a solid state detector is very similar to the movement of tons within the sensitive volume of gas filled detectors such as ionization chambers.
Common semiconductor detectors include Germanium, Cadmium Telluride, and Cadmium Zinc Telluride. Germanium Detectors produce much higher energy resolution than Nal, as shown in the discussion of resolution above. Germanium detectors produce the highest resolution commonly available today.
Calibration, Background:
It the spectrometer is used to identify a sample of unknown composition, its energy scale must be calibrated first. This is done using the peaks of a known source (like 137Cs or 60Co shown above). Since the channel number is proportional to energy, the channel scale can then be converted to an energy scale. If the size of the detector crystal is known, one can also perform an intensity calibration, so that not only the energies but also the intensities of an unknown source (or the amount of a certain isotope in the source) can be determined.
Because some radioactivity is present everywhere, one must also determine the “background”, i.e., the spectrum when no source is present. The background must then be subtracted from the actual measurement. By using lead absorbers around the apparatus, one can reduce the background.
Type # 2. X-ray Spectroscopy:
X-ray spectroscopy is a gathering name for several spectroscopic techniques for determining the electronic structure of materials by using X-ray excitation. When X-rays of sufficient frequency (energy) interact with a substance, inner shell electrons in the atom are excited to outer empty orbitals, or they may be removed completely, ionizing the atom.
The inner shell “hole” will then be filled by electrons from outer orbitals. The energy available in this de-excitation process is emitted as radiation (fluorescence) or will remove other less-bound electrons from the atom (Auger effect). The absorption or emission frequencies (energies) are characteristic of the specific atom.
In addition, for a specific atom small frequency (energy) variations occur which are characteristic of the chemical bonding. With a suitable apparatus, these characteristic X-ray frequencies or Auger electron energies can be measured. X-ray absorption and emission spectroscopy is used in chemistry and material sciences to determine elemental composition and chemical bonding.
X-ray crystallography is a scattering process; crystalline materials scatter X-rays at well-defined angles. If the wavelength of the incident X-rays is known, this allows calculation of the distances between planes of atoms within the crystal. The intensities of the scattered X-rays give information about the atomic positions and allow the arrangement of the atoms within the crystal structure to be calculated.
X-Ray Emission Spectroscopy:
Karl Manne Georg Siegbahn from Uppsala, Sweden (Nobel Prize 1924), who painstakingly produced numerous diamond-ruled glass diffraction gratings for his spectrometers, was one of the pioneers in developing X-ray emission spectroscopy (also called X-ray fluorescence spectroscopy). He measured the X-ray wavelengths of many elements to high precision, using high-energy electrons as excitation source.
Intense and wavelength-tunable X-rays are now typically generated with synchrotrons. In a material, the X-rays may suffer an energy loss compared to the incoming beam. This energy loss of the re-emerging beam reflects an internal excitation of the atomic system, an X-ray analogue to the well-known Raman spectroscopy that is widely used in the optical region.
In the X-ray region there is sufficient energy to probe changes in the electronic state (transitions between orbitals; this is in contrast with the optical region, where the energy loss is often due to changes in the state of the rotational or vibrational degrees of freedom). For instance, in the ultra-soft X-ray region (below about 1 keV), crystal field excitations give rise to the energy loss.
We may think of the photon-in-photon-out process as a scattering event. When the X-ray energy corresponds to the binding energy of a core level electron this scattering process is resonantly enhanced by many orders of magnitude. This type of X-ray emission spectroscopy is often referred to as resonant inelastic X-ray scattering (RIXS).
Due to the wide separation of orbital energies of the core levels, it is possible to select a certain atom of interest. The small spatial extent of core level orbitals forces the RIXS process to reflect the electronic structure in close vicinity of the chosen atom. Thus RIXS experiments give valuable information about the local electronic structure of complex systems, and theoretical calculations are relatively simple to perform.
Instrumentation:
There exist several efficient designs for analyzing an X-ray emission spectrum in the ultra-soft X-ray region. The figure of merit for such instruments is the spectral throughput, i.e., the product of detected intensity and spectral resolving power. Usually, it is possible to change the parameters within a certain range while keeping their product constant.
Grating Spectrometers:
Typically, the X-rays emerging from a sample must pass a source-defining slit, then optical elements (mirrors and/or gratings) disperse them by diffraction according to their wavelength and, finally, a detector is placed at their focal points.
Spherical Grating Mounts:
Henry Augustus Rowland (1848-1901) devised an instrument that allowed the use of a single optical element that combines diffraction and focusing: a spherical grating. Reflectivity of X -rays is low regardless of the used material and, therefore, grazing incidence upon the grating is necessary X-ray beams impinging on a smooth surface at a few degrees glancing angle of incidence undergo external total reflection which is taken advantage of to enhance the instrumental efficiency substantially.
Denote by R the radius of a spherical grating. Imagine a circle with half the radius R tangent to the centre of the grating surface. This small circle is called the Rowland circle. If the entrance slit is anywhere on this circle, then a beam passing the slit and striking the grating will be split into a secularly reflected beam, and beams of all diffraction orders, that come into locus at certain points on the same circle.
Plane Grating Mounts:
Similar as in optical spectrometers, a plane grating spectrometer first needs optic s that turns the divergent rays emitted by the X-ray source into a parallel beam. This may be achieved by using a parabolic mirror. The parallel rays emerging from this mirror strike a plane grating (with constant groove distance) at the same angle and are diffracted according to their wavelength.
A second parabolic mirror then collects the diffracted rays at a certain angle and creates an image on a detector. A spectrum within a certain wavelength range can be recorded simultaneously by using a 2-dimensional position sensitive detector such as a micro channel photomultiplier plate or an X-ray sensitive CCD chip (film plates are also possible to use).
Interferometers:
Instead of using the concept of multiple beam interference that gratings produce, one may simply let two rays interfere. By recording the intensity of two such co-linearly at some fixed point and changing their relative phase one obtains an intensity spectrum as a function of path length difference. One can show that this is equivalent to a Fourier transformed spectrum as a function of frequency.
The highest recordable frequency of such a spectrum is dependent on the minimum step size chosen in the scan and the frequency resolution (i.e., how well a certain wave can be defined in terms of its frequency) depends on the maximum path length difference achieved.
The latter feature allows a much more compact design for achieving higher resolution than for a grating spectrometer because X-ray wavelengths are small compared to attainable path length differences. Other important types of X-ray spectroscopic techniques include X-ray absorption spectroscopy and X-ray magnetic circular dichroism.
Type # 3. Ultraviolet-Visible Spectroscopy:
Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry (UV/VIS) involves the spectroscopy of photons and spectrophotometry. It uses light in the visible and adjacent to ultra violet (UV) and near infrared (NIR) ranges. In this region of energy space, molecules undergo electronic transitions.
All atoms absorb in the UV region because photons are energetic enough to excite outer electrons. If the frequency is high enough, photo-ionisation takes place. UV spectroscopy is also used in quantifying protein and DNA concentration as well as the ratio of protein to DNA concentration in a solution.
Several amino acids usually found in protein, such as tryptophan, absorb light in the 280 nm range and DNA absorbs light in the 260 nm range. For this reason, the ratio of 260/280 nm absorbance is a good general indicator of the relative purity of a solution in terms of these two macromolecules. Reasonable estimates of protein or DNA concentration can also be made this wav using Beer’s law.
UV/Vis spectroscopy is routinely used in the quantitative determination of solutions of transition metal ions and highly conjugated organic compounds. Solutions of transition metal ions can be coloured (i.e., absorb visible light) because electrons within the metal atoms can be excited from one electronic state to another.
The colour of metal ion solutions is strongly affected by the presence of other species, such as certain anions or ligands. For instance, the colour of a dilute solution of copper sulphate is very light blue; adding ammonia intensifies the colour and changes the wavelength of maximum absorption (λ max).
Organic compounds, especially those with a high degree of conjugation, also absorb light in the UV or visible regions of the electromagnetic spectrum. The solvents for these determinations are often water for water soluble compounds, or ethanol for organic soluble compounds. (Organic solvents may have significant UV absorption; not all sol vents are suitable for use in UV spectroscopy.
Ethanol absorbs very weakly at most wavelengths). While charge transfer complexes also give rise to colours, the colours are often too intense to be used for quantitative measurement. The Beer-Lambert law states that the absorbance of a solution is directly proportional to the solution’s concentration. Thus UV/Vis spectroscopy can be used to determine the concentration of a solution.
It is necessary to know how quickly the absorbance changes with concentration. This can be taken from references (tables of molar extinction coefficients), or more accurately, determined from a calibration curve. A UV/Vis spectrophotometer may be used as a detector for 11 PLC.
The presence of an analyte gives a response which can be assumed to be proportional to the concentration. For accurate results, the instrument’s response to the analyte in the unknown should be compared with the response to a standard; this is very similar to the use of calibration curves. The response (e.g., peak height) for a particular concentration is known as the response factor.
Beer-Lambert Law:
The Beer-Lambert law (also called the Beer-Lambert-Bouguer law or simply Beer’s law) is the linear relationship between absorbance and concentration of an absorber of electromagnetic radiation.
The general Beer-Lambert law is usually written as:
A = aλ × b × c,
where A is the measured absorbance, aλ is a wavelength-dependent absorptivity coefficient, b is the path length, and c is the analyte concentration. When working in concentration units of molarity, the Beer-Lambert law is written as;
A = ɛλ × b × c,
where is the wavelength-dependent molar absorptivity coefficient with units of M-1 cm-1. The λ subscript is often dropped with the understanding that a value for ɛ is for a specific wavelength. If multiple species that absorb light at a given wavelength are present in a sample, the total absorbance at that wavelength is the sum due to all absorbers:
A = (ɛ1 × b × c1) + (ɛ2 × b × c2) + … … ,
where the subscripts refer to the molar absorptivity and concentration of the different absorbing species that are present.
Theory:
Experimental measurements are usually made in terms of transmittance (T), which is defined as:
T = P/P0,
where P is the power of light after it passes through the sample and P0 is the initial light power. The relation between A and T is:
A = -log(T) = -log(P/P0).
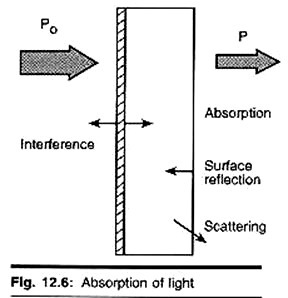
The figure shows the case of absorption of light through an optical filter and includes other processes that decrease the transmittance such as surface reflectance and scattering.
Modern absorption instruments can usually display the data as either transmittance, %-transmittance, or absorbance. An unknown concentration of an analyte can be determined by measuring the amount of light that a sample absorbs and applying Beer’s law. If the absorptivity coefficient is not known, the unknown concentration can he determined using a working curve of absorbance versus concentration derived from standards.
In analytical applications we often want to measure the concentration of an analyte independent of the effects of reflection, solvent absorption, or other interferences. The figure to the right shows the two transmittance measurements that are necessary to use absorption to determine the concentration of an analyte in solution. The top diagram is for solvent only and the bottom is for an absorbing sample in the same solvent.
In this example, Ps is the source light power that is incident on a sample, P is the measured light power after passing through the analyte, solvent, and sample holder, and P0 is the measured light power after passing through only the solvent and sample holder. The measured transmittance in this case is attributed to only the analyte.
Depending on the type of instrument, the reference measurement (top diagram) might be made simultaneously with the sample measurement (bottom diagram) or a reference measurement might be saved on I computer to generate the full spectrum.
Derivation of the Beer-Lambert Law:
The Beer-Lambert law can be derived from an approximation for the absorption coefficient tor a molecule by approximating the molecule by an opaque disk whose cross-sectional area, σ, represents the effective area seen by a photon of frequency w. If the frequency of the light is far from resonance, the area is approximately 0, and if w is close to resonance the area is a maximum. Taking an infinitesimal slab, dz, of sample:
I0 is the intensity entering the sample at z=0, Iz is the intensity entering the infinitesimal slab at z, dl is the intensity absorbed in the slab, and I is the intensity of light leaving the sample. Then, the total opaque area on the slab due to the absorbers is σ * N * A * dz. Then, the fraction of photons absorbed will be σ * N * A * dz / A; so,
Limitations of the Beer-Lambert Law:
The linearity of the Beer-Lambert law is limited by chemical and instrumental factors. Causes of nonlinearity include:
i. Deviations in absorptivity coefficients at high concentrations (>0.01M) due to electrostatic interactions between molecules in close proximity
ii. Scattering of light due to particulates in the sample
iii. Fluorescence or phosphorescence of the sample
iv. Changes in refractive index at high analyte concentration
v. Shifts in chemical equilibria as a function of concentration
vi. Non-monochromatic radiation, deviations can be minimized by using a relatively flat part of the absorption spectrum such as the maximum of an absorption band
vii. Stray light.
Ultraviolet-Visible Spectrophotometer:
The UV-Vis spectral range is approximately 190 to 900 nm, as defined by the working range of typical commercial UV-Vis spectrophotometers. The short-wavelength limit for simple UV-Vis spectrometers is the absorption of ultraviolet wavelengths less than 180 nm by atmospheric gases. Purging a spectrometer with nitrogen gas extends this limit to 175 nm.
Working beyond 175 nm requires a vacuum spectrometer and a suitable UV light source. The long-wavelength limn is usually determined by the wavelength response of the detector in the spectrometer. High end commercial UV Vis spectrophotometers extend the measurable spectral range into the NIR region as tar as 3300 nm.
The light source is usually a deuterium discharge lamp tor UV measurements and a tungsten halogen lamp for visible and NIR measurements. The instruments automatically swap lamps when scanning between the UV and visible regions. The wavelengths of these continuous light sources are typically dispersed by a holographic grating m a single or double monochromator or spectrograph.
The spectral band-pass is then determined by the monochromator slit width or by the array-element width in array-detector spectrometers. Spectrometer designs and optical components are optimized to reject stray light, which is one of the limiting factors in quantitative absorbance measurements.
The detector in single-detector instruments is a photodiode, phototube, or photomultiplier tube (PMT). UV-Vis-NIR spectrometers utilize a combination of a PMT and a Peltier-cooled PbS IR detector. The light beam is redirected automatically to the appropriate detector when scanning between the visible and NIR regions. The diffraction grating and instrument parameters such as slit width can also change.
Most commercial UV-Vis absorption spectrometers use one of three overall optical designs: a fixed or scanning spectrometer with a single light beam and sample holder, a scanning spectrometer with dual light beams and dual sample holders for simultaneous measurement of P and P0, or a non-scanning spectrometer with an array detector for simultaneous measurement of multiple wavelengths.
In single-beam and dual-beam spectrometers, the light from a lamp is dispersed before- reaching the sample cell. In an array-detector instrument, all wavelengths pass through the sample and the dispersing element is between the sample and the array detector.
Instrument Designs:
i. Single-beam UV-Vis spectrophotometer
ii. Dual-beam UV-Vis spectrophotometer
iii. Array detector UV-Vis spectrophotometer
Single-Beam UV-Vis Spectrophotometer:
Single-Beam spectrophotometers are often sufficient for making quantitative absorption measurements in the UV-Vis spectral region. The concentration of an analyte in solution can be determined by measuring the absorbance at a single wavelength and applying the Beer-Lambert Law.
Single-beam spectrophotometers can utilize a fixed wavelength light source or a continuous source. The simplest instruments use a single-wavelength light source, such as a light-emitting diode (THD), a sample container, and a photodiode detector.
Instruments with a continuous source have a dispersing element and aperture or slit to select a single wavelength before the light passes through the sample cell (see schematic below). In either type of single-beam instrument, the instrument is calibrated with a reference cell containing only solvent to determine the P0 value necessary for an absorbance measurement.
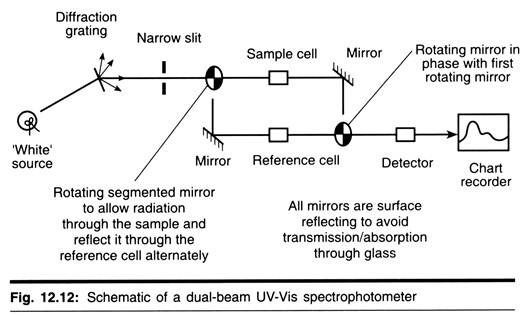
Dual-Beam UV-Vis Spectrophotometer:
In single-beam UV-Vis absorption spectroscopy, obtaining a spectrum requires manually measuring the transmittance of the sample and solvent at each wavelength. The double-beam design greatly simplifies this process by measuring the transmittance of the sample and solvent simultaneously.
The detection electronics can then manipulate the measurements to give the absorbance. The dual-beam design greatly simplifies this process by simultaneously measuring P and P0 of the sample and reference cells, respectively. Most spectrometers use a mirrored rotating chopper wheel to alternately direct the light beam through the sample and reference cells. The detection electronics or software program can then manipulate the P and P0 values as the wavelength seal to produce the spectrum of absorbance or transmittance as a function of wavelength.
Array-Detector Spectrophotometer:
Array-detector spectrophotometers allow rapid recording of absorption spectra. Dispersing the source light after it passes through a sample allows the use of an array detector to simultaneously record the transmitted light power at multiple wavelengths. There are a large number of applications where absorbance spectra must be recorded very quickly.
Some examples include HPLC detection, process monitoring, and measurement of reaction kinetics. These spectrometers use photodiode arrays (PDAs) or charge-coupled devices (CCDs) as the detector. The spectral range of these array detectors is typically 200 to 1000 nm. The light source is a continuum source such as a tungsten lamp. All wavelengths pass through the sample.
The light is dispersed by a diffraction grating after the sample and the separated wavelengths fall on different pixels of the array detector. The resolution depends on the grating, spectrometer design, and pixel size, and is usually fixed for a given instrument. Besides allowing rapid spectral recording, these instruments are relatively small and robust.
Portable spectrometers have been developed that use optical fibers to deliver light to and from a sample. These instruments use only a single light beam, so a reference spectrum is recorded and stored in memory to produce transmittance or absorbance spectra after recording the sample spectrum.
Ultraviolet-visible Spectrum:
An ultraviolet-visible spectrum is essentially a graph of light absorbance versus wavelength in a range of ultraviolet or visible regions. Such a spectrum can often be produced directly by a more sophisticated spectrophotometer, or the data can be collected one wavelength at a time by simpler instruments. Wavelength is often represented by the symbol X.
Similarly, for a given substance, a standard graph of the extinction coefficient (ɛ) vs. wavelength (λ) may be made or used if one is already available. Such a standard graph would be effectively “concentration-corrected” and thus independent of concentration. For the given substance, the wavelength at which maximum absorption in the spectrum occurs is called pronounced “Lambda-max”.
The Woodward-Fieser rules are a set of empirical observations which can be used to predict λmax, the wavelength of the most intense UV/Vis absorption, for conjugated organic compounds such as dienes and ketones. This spectrum can be used qualitatively to identify components in a sample as each component has their own unique absorbance spectrum (like a fingerprint).
Type # 4. Fluorescence Spectroscopy:
Fluorescence spectroscopy or fluorometry or spectrofluorimetry is a type of electromagnetic spectroscopy which analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit light of a lower energy; typically, but not necessarily, visible light. A complementary technique is absorption spectroscopy. Devices that measure fluorescence are called fluorometers or fluorimeters.
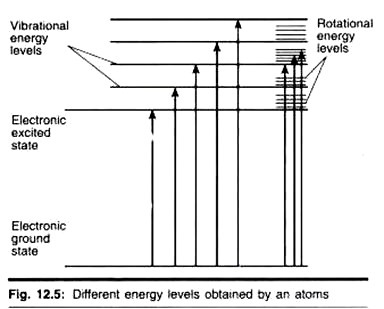
Molecules have various states referred to as energy levels. Fluorescence spectroscopy is primarily concerned with electronic states and vibrational states. Generally, the species being examined will have a ground electronic state (a low energy state) of interest, and an excited electronic state of higher energy. Each of these electronic states has various vibrational states.
Photons of light are small “packets” of energy, each with an energy proportional to its frequency; photons of high frequency light have higher energy than those of low frequency light. These can be absorbed by molecules, with the molecule gaining the energy of the photon, or emitted by molecules, with the photon carrying some of the energy of the molecule away.
In fluorescence spectroscopy, the species is first excited, by absorbing a photon of light, from its ground electronic state to one of the various vibrational states in the excited electronic state. Collisions with other molecules cause the excited molecule to lose vibrational energy until it reaches the lowest vibrational state of the excited electronic state.
The molecule then drops down to one of the various vibrational levels of the ground electronic state again, emitting a photon in the process. As molecules may drop down into any of the vibrational levels of this ground state, the photons will have different energies, and thus frequencies. Therefore, by analysing the different frequencies of light emitted in fluorescent spectroscopy, the structure of these different vibrational levels can be determined.
Typically, the different frequencies of fluorescent light emitted by a sample, when the excited light is held at a constant wavelength are measured. This is called an emission spectrum. An excitation spectrum measured by recording the sum of the fluorescent light emitted at all frequencies a: function of the frequency of the monochromatic incident light.
Instrumentation:
Two general types of instruments exist:
1. Filter fluorometers use filters to isolate the incident light and fluorescent light.
2. Spectro-fluorometers use diffraction grating monochromators (two) to isolate the incident light and fluorescent light.
Both types utilize the following scheme:
The light from an excitation source passes through a filter or monochromator, and passes through the sample. Here some of it probably is absorbed, making some of the molecules in the sample fluoresce. A part of the fluorescent light is then focused on a filter or monochromator, which often is placed at a 90° angle to the excitation light.
The light is then detected by a detection device. Various light sources may be used as excitation sources; including lasers, photodiodes, and lamps; xenon arcs and mercury vapour lamps in particular. A laser only emits light of high irradiance at a very narrow wavelength interval, typically under 0.01 nm, which makes an excitation monochromator or filter unnecessary.
The downside is that the wavelength of a laser cannot be changed much. A mercury vapour lamp is a line lamp, meaning it emits light near peak wavelengths contrary to the Xe arc, which have a continuous emission spectrum with nearly constant intensity in the range from 300-800 nm and have a sufficient irradiance tor measurement down to just above 200 nm.
Filters and/or monochromators may be used in fluorimeters. A monochromator transmits light of an adjustable wavelength with an adjustable tolerance. The most common type of monochromator utilizes diffraction grating, that is, collimated light enters a grating and exits with a different angle depending on the wavelength.
The monochromator can then select which wavelengths to transmit. For allowing anisotropy measurements the addition of two polarization filters are necessary: One after the excitation monochromator or filter, and one before the emission monochromator or filter. The fluorescence is most often measured at a 90° angle relative to the excitation light.
This geometry is used instead of placing the sensor at the line of the excitation light at a 180° angle in order to avoid interference of the transmitted excitation light. No monochromator is perfect and it will transmit some stray light, that is, light with other wavelengths than the targeted. An ideal monochromator would only transmit light in the specified range and have a high wavelength-independent transmission.
When measuring at a 90° angle, only the light scattered by the sample causes stray light. This results in a better signal-to-noise ratio, and lowers the detection limit by approximately a factor 10000, when compared to the 180° geometry. Furthermore, the fluorescence can also be measured from the front, which is often done for turbid samples.
The detector can either be single-channeled or multi-channeled. The single channeled detector can only detect the intensity of one wavelength at a time, while the multi-channeled one detects tin intensity at all wavelengths simultaneously, making the emission monochromator or filter unnecessary. The different types of detectors have both advantages and disadvantages.
The most versatile fluorimeters with dual monochromators and a continuous excitation light source can record both an excitation spectrum and a fluorescence spectrum. When measuring fluorescence spectra, the wavelength of the excitation light is kept constant, preferably at a wavelength of high absorption, and the emission monochromator scans the spectrum.
For measuring excitation spectra, the wavelength passing though the emission filter or monochromator is kept constant and the excitation monochromator is scanning. The excitation spectrum generally is identical to the absorption spectrum as the fluorescence intensity is proportional to the absorption.
Analysis of Data:
At low concentrations the fluorescence intensity will generally be proportional to the concentration of the fluorophore. Contrary to UV/Vis spectroscopy ‘standard’, device-independent spectra are not easily attained, however. Several factors influence and distort the spectra or represents factors that need to be corrected to attain ‘true’ spectra.
The different types of distortions will here be classified as being either instrumentally or sample related. Firstly, the distortion arising from the instrument is discussed. As a start, the light source intensity and wavelength characteristics varies over time during each experiment and between each experiment. Furthermore, no lamp has a constant intensity at all frequencies.
To correct this, a beam splitter can be applied after the excitation monochromator or filter to direct a portion of the light to a reference detector. Additionally, the transmission efficiency of monochromators and filters has to be taken into account. These may also change over time. The transmission efficiency of the monochromator also varies depending on wavelength.
This is the reason that an optional reference detector should be placed after the excitation monochromator or filter. The percentage of the fluorescence picked up by the detector is also dependent upon the system. Furthermore, the detector quantum efficiency, that is, the percentage of photons detected, varies between different detectors, with wavelength and with rime, as the- detector inevitably deteriorates.
Correction of all these instrumental factors for getting a ‘standard’ spectrum is a tedious process, which is only applied in practice when it is strictly necessary. This is the case when measuring the quantum yield or when finding the wavelength with the highest emission intensity tor instance. Distortions arise from the sample as well.
Therefore some aspects of the sample must be taken into account too. Firstly, photodecomposition may decrease the intensity of fluorescence over time. Scattering of light must also be taken into account. The most significant types of scattering in this context are Rayleigh and Raman scattering. Light scattered by Rayleigh scattering has the same wavelength as the incident light, whereas in Raman scattering the scattered light changes wavelength usually to longer wavelengths.
Raman scattering is the result of a virtual electronic state induced by the excitation light. From this virtual state, the molecules may relax back to a vibrational level other than the vibrational ground state. In fluorescence spectra, it is always seen at a constant wave number difference relative to the excitation wave number, e.g., the peak appears at a wave number 3600 cm-1 lower than the excitation light in water.
Other aspects to consider are the inner filter effects. These include reabsorption. Reabsorption happens because another molecule or part of a macromolecule absorbs at the wavelengths at which the fluorophore emits radiation. If this is the case, some or all of the photons emitted by the fluorophore may be absorbed again.
Another inner filter effect occurs because of high concentrations of absorbing molecules, including the fluorophore. The result is that the intensity of the excitation light is not constant throughout the solution—only a small percentage of the excitation light reaches the fluorophores that are visible for the detection system. The inner filter effects change the spectrum and intensity of the emitted light and they must, therefore, be considered when analysing the emission spectrum of fluorescent light.
Applications:
It is used in, among others, biochemical, medical, and chemical research fields, for analyzing organic compounds. There has been report of its use in differentiating malign skin tumours from benign: a kind of tumour cells.
Type # 5. Circular Dichroism Spectroscopy:
Circular dichroism (CD) spectroscopy is a type of absorption spectroscopy that can provide information on the structures of many types of biological macromolecules. Circular Dichroism is the difference between the absorption of left and right handed circularly-polarised light and is measured as a function of wavelength. CD is measured as a quantity called mean residue ellipticity, whose units are degrees-cm2/dmol.
Chiral or asymmetric molecules produce a CD spectrum because they absorb left and right handed polarised light to different extents and thus are considered to be “optically active”. Biological macromolecules such as proteins, polysaccharides and DNA are composed of optically active elements and because they can adopt different types of three- dimensional structures, each type of molecule produces distinct CD spectra.
The wavelengths of light that are most useful for examining the structures of proteins and DNA are in the ultraviolet (UV) or vacuum ultraviolet (VUV) ranges (from 160 to 300 nm) because these are the regions of the electronic transitions of the peptide backbone and side chains in proteins and the purine and pyrimidine bases in DNA. Typically the range of data collected on a commercially available CD instrument will be between 190 nm and 300 nm.
Different types of protein secondary structures (helices, sheets, turns and coils) give rise to different CD spectra.
Since the spectrum of a protein is directly related to its secondary structure content, the spectrum will be a linear combination of each of these “reference” spectra, weighted by the traction of the type of secondary structure. Therefore, it is possible to mathematically extract the secondary- structure information for an unknown protein from its CD spectrum. Some of the advantages of CD spectroscopy are that it requires only very small amounts of material (100 micrograms or less) and measurements can be done very quickly (in 30 minutes or less).
CD spectroscopy has been used to monitor:
(1) Secondary structure,
(2) Conformational changes,
(3) Environmental effects,
(4) Protein folding and denaturation, and
(5) Dynamics.
Type # 6. Atomic Spectroscopy:
Atomic spectroscopy is the determination of elemental composition by its electromagnetic or mass spectrum. Atomic spectroscopy is closely related to other forms of spectroscopy. It can be divided by atomization source or by the type of spectroscopy used. In the latter case, the main division is between optical and mass spectrometry.
Mass spectrometry generally gives significantly better analytical performance, but is also significantly more complex. This complexity translates into higher purchase costs, higher operational costs, more operators training, and a greater number of components that can potentially fail. Because optical spectroscopy is generally less expensive and has performance adequate for many tasks, it is far more common. Atomic absorption spectrometers arc one of the most commonly sold and used analytical devices.
1. Optical Spectrometry, and
2. Atomic Mass Spectrometry.
1. Optical Spectrometry:
Electrons exist in energy levels within an atom. These levels have well defined energies and electrons moving between them must absorb or emit energy equal to the difference between them. In optical spectroscopy, the energy absorbed to move an electron to a more energetic level and/or the energy emitted as the electron moves to a less energetic energy level is in the form of a photon (a particle of light). Because this energy is well-defined, an atom’s identity (i.e., what element it is) can be identified by the energy of this transition.
The wavelength of light can be related to its energy. It is usually easier to measure the wavelength of light than to directly measure its energy. Optical spectroscopy can be further divided into absorption, emission, and fluorescence.
i. Atomic-Absorption Spectroscopy (AA):
Atomic absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids, the analyte atoms or ions must be vaporized in a flame or graphite furnace. The atoms absorb ultraviolet or visible light and make transitions to higher electronic energy levels. The analyte concentration is determined from the amount of absorption.
Applying the Beer-Lambert law directly in AA spectroscopy is difficult due to variations in the atomization efficiency from the sample matrix, and non-uniformity of concentration and path length of analyte atoms (in graphite furnace AA). Concentration measurements are usually determined from a working curve after calibrating the instrument with standards of known concentration.
Instrumentation:
The light source is usually a hollow-cathode lamp of the element that is being measured. Lasers are also used in research instruments. Since lasers are intense enough to excite atoms to higher energy levels, they allow AA and atomic fluorescence measurements in a single instrument. The disadvantage of these narrow-band light sources is that only one element is measurable at a time.
AA spectroscopy requires that the analyte atoms be in the gas phase. Ions or atoms in a sample must undergo de-solvation and vaporization in a high-temperature source such as a flame or graphite furnace. Flame AA can only analyze solutions, while graphite furnace AA can accept solutions, slurries, or solid samples.
Flame AA uses a slot type burner to increase the path length, and, therefore, to increase the total absorbance (Beer-Lambert law). Sample solutions are usually aspirated with the gas flow into a nebulizing/mixing chamber to form small droplets before entering the flame. The graphite furnace has several advantages over a flame.
It is a much more efficient atomizer than a flame and it can directly accept very small absolute quantities of sample. It also provides reducing environment for easily oxidized elements. Samples are placed directly in the graphite furnace and the furnace is electrically heated in several steps to dry the sample, ash organic matter, and vaporize the analyte atoms.
AA spectrometers use monochromators and detectors for UV and visible light. The main purpose of the monochromator is to isolate the absorption line from back ground light due to interferences. Simple dedicated AA instruments often replace the monochromator with a band-pass interference filter. Photomultiplier tubes arc the most common detectors for AA spectroscopy.
ii. Atomic Emission Spectroscopy (AES, OES):
Atomic emission spectroscopy (AES or OES [optical emission spectroscopy]) uses quantitative measurement of the optical emission from excited atoms to determine analyte concentration. Analyte atoms in solution are aspirated into the excitation region where they are de-solvated, vaporized, and atomized by a flame, discharge, or plasma.
These high-temperature atomization sources provide sufficient energy to promote the atoms into high energy levels. The atoms decay back to lower levels by emitting light. Since the transitions are between distinct atomic energy levels, the emission lines in the spectra are narrow. The spectra of samples containing many elements can be very congested, and spectral separation of nearby atomic transitions requires a high-resolution spectrometer.
Since all atoms in a sample are excited simultaneously, they can be detected simultaneously using a polychromator with multiple detectors. This ability to simultaneously measure multiple elements is a major advantage of AES compared to atomic-absorption (AA) spectroscopy.
Instrumentation:
As in AA spectroscopy, the sample must be converted to free atoms, usually in a high temperature excitation source. Liquid samples are nebulized and carried into the excitation source by a flowing gas. Solid samples can be introduced into the source by slurry or by laser ablation of the solid sample in a gas stream. Solids can also lie directly vaporized and excited by a spark between electrodes or by a laser pulse. The excitation source must de-solvate, atomize, and excite the analyte atoms.
The various excitation sources used are:
i. Direct-current plasma (DCP)
ii. Flame
iii. Inductively-coupled plasma (ICP)
iv. Laser induced breakdown (LIBS)
v. Laser induced plasma
vi. Microwave-induced plasma (MIP)
vii. Spark or arc.
Since the atomic emission lines are very narrow, a high resolution polychromator is needed to selectively monitor each emission line.
iii. Atomic-Fluorescence Spectroscopy(AFS):
Atomic fluorescence is the optical emission from gas-phase atoms that have been excited to higher energy levels by absorption of electromagnetic radiation. The main advantage of fluorescence detection compared to absorption measurements is the greater sensitivity achievable because the fluorescence signal has a very low background.
The resonant excitation provides selective excitation of the analyte to avoid interferences. AFS is useful to study the electronic structure of atoms and to make quantitative measurements. Analytical applications include flames and plasmas diagnostics, and enhanced sensitivity in atomic analysis.
Instrumentation:
Analysis of solutions or solids requires that the analyte atoms be de-solvated, vaporized, and atomized at a relatively low temperature in a heat pipe, flame, or graphite furnace. A hollow cathode lamp or laser provides the resonant excitation to promote the atoms to higher energy levels. The atomic fluorescence is dispersed and detected by monochromators and photomultiplier tubes, similar to atomic-emission spectroscopy instrumentation.
2. Atomic Mass Spectrometry:
Atomic mass spectrometry is similar to other types of mass spectrometry in that it consists of an ion source, a mass analyzer, and a detector. Atoms’ identities are determined by their mass-to-charge ratio (via the mass analyzer) and their concentrations are determined by the number of ion detected.
Although considerable research has gone into customizing mass spectrometers for atomic ion sources, it is the ion source that differs most from other forms of mass spectrometry. These ion sources must also atomize samples, or an atomization step must take place before ionization. Atomic ion sources are generally modifications of atomic optical spectroscopy atom sources.
Ion and Atom Sources:
Sources can be adapted in many ways, but the lists below gives the general uses of a number of sources. Of these, flames are the most common due to their low cost and their simplicity. Although significantly less common, inductively-coupled plasmas, especially when used with mass spectrometers, are recognized for their outstanding analytical performance and their versatility. For all atomic spectroscopy, a sample must be vaporized and atomized. For atomic mass spectrometry, a sample must also be ionized.
Vaporization, atomization, and ionization are often, but not always, accomplished with a single source. Alternatively, one source may be used to vaporize a sample while another is used to atomize (and possibly ionize). An example of this would be laser ablation inductively-coupled plasma atomic emission spectrometry, where a laser is used to vaporize a solid sample and an inductively-coupled plasma is used to atomize the vapour. With the exception o flames and graphite furnaces, which are most commonly used for atomic absorption spectroscopy, most sources are used primarily for atomic emission spectroscopy.
Liquid-sampling Sources Include:
i. Flames and sparks (atom source)
ii. Inductively-coupled plasma (atom and ion source)
iii. Graphite furnace (atom source)
iv. Microwave plasma (atom and ion source)
v. Direct-current plasma (atom and ion source)
Solid-sampling Sources Include:
i. Lasers (atom and vapour source)
ii. Glow discharge (atom and ion source)
iii. Arc (atom and ion source)
iv. Spark (atom and ion source)
v. Graphite furnace (atom and vapour source)
Gas-sampling Sources Include:
i. Flame (atom source)
ii. Inductively-coupled plasma (atom and ion source)
iii. Microwave plasma (atom and ion source)
iv. Direct-current plasma (atom and ion source)
v. Glow discharge (atom and ion source)
Type # 7. Infrared Spectroscopy:
The infrared region of the electromagnetic spectrum extends from 14,000 cm-1 to 10 cm-1. The region of most interest for chemical analysis is the mid-infrared region (4,000 cm-1 to 400 cm-1) which corresponds to changes in vibrational energies within molecules. The tar infrared region (400 cm-1 to 10 cm-1) is useful for molecules containing heavy atoms such as inorganic compounds but requires rather specialized experimental techniques. It is rarely, if ever, possible to identify an unknown compound by using IR spectroscopy alone.
Its principal strengths are:
(i) it is a quick and relatively cheap spectroscopic technique,
(ii) it is useful for identifying certain functional groups in molecules and
(iii) an IR spectrum of a given compound is unique and can, therefore, serve as fingerprint for this compound.
Molecular Vibrations:
All molecules vibrate, even at a temperature of absolute zero. In general, a polyatomic molecule with N atoms has 3Ñ6 distinct vibrations. Each of these vibrations has an associated set of quantum states and in IR spectroscopy the IR radiation induces a jump from the ground (lowest) to the first excited quantum state. Although approximate, each vibration in a molecule can be associate d with motion in a particular group.
A simple example is methanal, whose 6 vibrations involve the following motions:
Clearly, even tor this simple molecule, a description of the vibrations is quite complicated and this complexity rapidly increases as the size of the molecule increases. Fortunately, as we will see, it is almost never necessary to be able to picture all the molecular vibrations for IR spectroscopy to be useful.
Infrared Activity:
Not all possible vibrations within a molecule will result in an absorption band in the infrared region. To be infrared active the vibration must result in a change of dipole moment during the vibration. This means that for homo-nuclear diatomic molecules such as Hydrogen (H2), Nitrogen (N2) and Oxygen (O2), no infrared absorption is observed, as these molecules have zero dipole moment and stretching of the bonds will not produce one.
For hetero-nuclear diatomic molecule such Carbon Monoxide (CO) and Hydrogen Chloride (HCL), which do possess a permanent dipole moment, infrared activity occurs because stretching of this bond leads to a change in dipole moment.
It is important to remember that it is not necessary for a compound to have a permanent dipole moment to be infrared active. In the case of Carbon Dioxide (CO2) the molecule is linear and Centro symmetric and therefore does not have a permanent dipole moment. This means that the symmetric stretch will not be infrared active. However, in the case of the asymmetric stretch a dipole moment will be periodically produced and destroyed resulting in a changing dipole moment and, therefore, infrared activity.
The Fingerprint Region:
The fact that there are many different vibrations even within relatively simple molecules means that the infrared spectrum of a compound usually contains a large number or peaks, many of which will be impossible to confidently assign to vibration of a particular group.
Particularly notable is the complex pattern of peaks below 1500 cm-1 which arc very difficult to assign. However, this complexity has an important advantage; in that it can serve as a fingerprint for a given compound. Consequently, by referring to known spectra, the region can be used to identify a compound.
Interpretation of Spectra:
To obtain a more detailed interpretation of an IR spectrum it is necessary to refer to correlation charts and tables of infrared data. There are many different tables available for reference and a brief summary is given below for some of the main groups. When assigning peaks to specific groups in the infrared region it is usually the stretching vibrations which are most useful.
Broadly speaking, these can be divided into four regions:
3700 – 2500 cm-1 Single bonds to hydrogen
2300 – 2000 cm-1 Triple bonds
1900 – 1500 cm-1 Double bonds
1400 – 650 cm-1 Single bonds (other than hydrogen)
It should also be noted that the region 1650 – 650 cm-1 contains peaks due to bending vibrations but it is rarely possible to assign a specific peak to a specific group.
Sample Preparation:
There are a variety of techniques for sample preparation dependent on the physical form of the sample to be analysed.
Solids:
There are two main methods for sample preparation involving the use of Nujol mull or potassium bromide disks. However, there is also a third option of preparing a solution in a suitable solvent (not infrared active in the region of interest).
Nujol Mull:
The sample is ground using an agate mortar and pestle to give a very fine powder. A small amount is then mixed with nujol to give a paste and several drops of this paste arc then applied between two sodium chloride plates (these do not absorb infrared in the region of interest). The plates are then placed in the instrument sample holder ready for scanning.
Potassium Bromide Disk:
A very small amount of the solid (approximately 12 mg) is added to pure potassium bromide powder (approximately 200 mg) and ground up as fine as possible. This is then placed in a small die and put under pressure mechanically. The pressure is maintained for several minutes before removing the die and the KBr disk formed. The disk is then placed in a sample holder ready for scanning.
The success of this technique is dependent on the powder being ground as fine as possible to minimise infrared light scattering off the surface of the particles. It is also important that the sample be dry before preparation. KBr has no infrared absorption in the region 400-650 cm-1.
Thin Films:
The infrared spectrum of a thin film can be easily obtained by placing a sample in a suitable holder, such as a card with a slot cut for the sample window. This method is often used for checking the calibration of an instrument with a polystyrene sample as the bands produced by this material are accurately known.
Liquids:
This is possibly the simplest and most common method of sample preparation. A drop of the sample is placed between two potassium bromide or sodium chloride circular plates to produce a thin capillary film. The plates are then placed in a holder ready for analysis.
Gases:
lb obtain an infrared spectrum of a gas requires the use of a cylindrical gas cell with windows at each end composed of an infrared inactive material such as KBr, NaCl or CaF2. The cell usually has an inlet and an outlet ports with a tap to enable the cell to be easily filled with the gas to be analysed.
Example Spectra:
1. Alcohols:
n-Butanol CH3CH2CH2CH2OH
Carboxylic Acids:
Ethanoic Acid CH3COOH
Aldehydes and Ketones:
Butanone CH3COCH2CH3
Esters:
Ethyl Ethanoate CH3COOC2H5
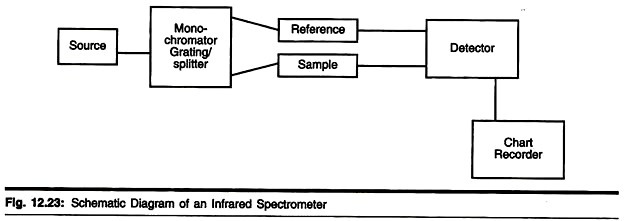
Instrumentation:
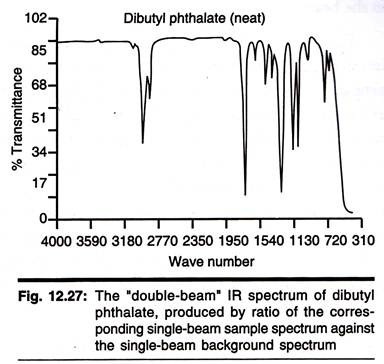
The Double Beam Infrared Spectrometer
This instrument uses a source of infrared radiation such as a nichrome wire or cooled rod of silicon carbide to produce a range of frequencies which are then separated into individual frequencies using a monochromator diffraction grating. The beam produced is then split into two and one passes through the sample whilst the other is used as a reference beam.
The two beams then converge on the detector which measures the difference in intensity and then sends a proportional signal to the recorder. The resulting plot is a measure of transmission against frequency which is usually plotted as wave- number (cm-1).
Wave number can be related to the frequency by using the following equations:
The units for wavenumber are cm-1, therefore, wavelength should be converted to cm rather than metres for this calculation and wavelength is related to frequency as:
Velocity of light (c) =
Units:
Wave number cm-1
Wavelength m
Frequency Hz
Velocity of light ms-1
Fourier Transform Spectrometers:
Fourier transform spectrometers have recently replaced dispersive instruments for most applications due to their superior speed and sensitivity. They have greatly extended the capabilities of infrared spectroscopy and have been applied to many areas that are very difficult or nearly impossible to analyze by dispersive instruments. Instead of viewing each component frequency sequentially, as in a dispersive IR spectrometer, all frequencies are examined simultaneously in Fourier transform infrared (FTIR) spectroscopy.
Instrumentation:
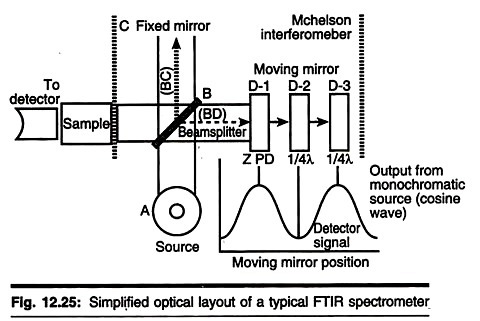
There are three basic spectrometer components in an FT system: radiation source, interferometer, and detector. A simplified optical layout of a typical FTIR spectrometer is illustrated in the con concerned figure below. The same types of radiation sources are used for both dispersive and Fourier transforms spectrometers. However, the source is more often water-cooled in FTIR instruments to provide better power and stability.
In contrast, a completely different approach is taken in an FTIR spectrometer to differentiate and measure the absorption at component frequencies. The monochromator is replaced by an interferometer, which divides radiant beams, generates an optical path difference between the beams, and then recombines them in order to produce repetitive interference signals measured as a function of optical path difference by a detector. As its name implies, the interferometer produces interference signals, which contain infrared spectral information generated after passing through a sample.
The most commonly used interferometer is a Michelson interferometer. It consists of three active components: a moving mirror, a fixed mirror, and a beam splitter. The two mirrors arc- perpendicular to each other. The beam splitter is a semi reflecting device and is often made by depositing a thin film of germanium onto a flat KBr substrate. Radiation from the broadband IR source is collimated and directed into the interferometer, and impinges on the beam splitter.
At the beam splitter, half the IR beam is transmitted to the fixed mirror and the remaining half is reflected to the moving mirror. After the divided beams are reflected from the two mirrors, they are recombined at the beam splitter. Due to changes in the relative position of the moving mirror to the fixed mirror, an interference pattern is generated. The resulting beam then passes through the sample and is eventually focused on the detector.
For an easier explanation, the detector response for a single-frequency component from the IR source is first considered. This simulates an idealized situation where the source is monochromatic, such as a laser source. As previously described, differences in the optical paths between the two split beams are created by varying the relative position of moving mirror to the fixed mirror.
It the two arms of the interferometer are of equal length, the two split beams travel through the exact same path length. The two beams are totally in phase with each other; thus, they interfere constructively and lead to a maximum in the detector response. This position of the moving mirror is called the point of zero path difference (ZPD).
When the moving mirror travels in either direction by the distance λ/4, the optical path (beam splitter-mirror-beamsplitter) is changed by 2 (λ/4), or λ/2 Th. two beams are 180° out of phase with each other, and thus interfere destructively. As the moving mirror travels another λ/4, the optical path difference is now 2 (λ/2), or λ.
The two beams are again in phase with each other and result in another constructive interference. When the mirror is moved at a constant velocity, the intensity of radiation reaching the detector varies in a sinusoidal manner to produce the interferogram output shown in Fig. 12.25. The interferogram is the record of the interference signal.
It is actually a time domain spectrum and records the detector response changes versus time within the mirror scan. If the sample happens to absorb at this frequency, the amplitude or the sinusoidal wave is reduced by an amount proportional to the amount of sample in the beam.
Extension of the same process to three component frequencies results in a more complex interferogram, which is the summation of three individual modulated waves. In contrast to this simple, symmetric interferogram, the interferogram produced with a broadband IR source displays extensive interference patterns.
It is a complex summation of superimposed sinusoidal waves, each wave corresponding to a single frequency. When this IR beam is directed through the sample, the amplitudes of a set of waves are reduced by absorption if the frequency of this set of wave is the same as one of the characteristic frequencies of the sample.
The interferogram contains information over the entire IR region to which the detector is responsive. A mathematical operation known as Fourier transformation converts the interferogram (a time domain spectrum displaying intensity versus time within the mirror scan) to the final IR spectrum, which is the familiar frequency domain spectrum showing intensity versus frequency. This also explains how the term Fourier transform infrared spectrometry is created.
The detector signal is sampled at small, precise intervals during the mirror scan. The sampling rate is controlled by an internal, independent reference, a modulated monochromatic beam from helium neon (HeNe) laser focused on a separate detector.
The two most popular detectors for a FTIR spectrometer are deuterated triglycine sulphate (DTGS) and mercury cadmium telluride (MCT). The response times of many detectors (for example, thermocouple and thermistor) used in dispersive IR instruments are too slow for the rapid scan times (1 sec or less) of the interferometer. The DTGS detector is a pyro-electric detector that delivers rapid responses because it measures the changes in temperature rather than the value of temperature.
The MCT detector is a photon (or quantum) detector that depends on the quantum nature of radiation and also exhibits very fast responses. Whereas DTGS detectors operate at room temperature, MCT detectors must be maintained at liquid nitrogen temperature (77°K) to be effective. In general, the MCT detector is faster and more sensitive than the DTGS detector.
Spectrometer Design:
The basic instrument design is quite simple. The IR radiation from a broadband source is first directed into an interferometer, where it is divided and then recombined after the split beams travel different optical paths to generate constructive and destructive interference. Next, the resulting beam passes through the sample compartment and reaches to the detector. Most bench top FTIR spectrometers are single-beam instruments. Unlike double-beam grating spectrometers, single-beam FTIR does not obtain transmittance or absorbance IR spectra in real time.
FTIR Advantages:
FTIR instruments have distinct advantages over dispersive spectrometers:
i. Better speed and sensitivity (Felgett advantage). A complete spectrum can be obtained during a single scan of the moving mirror, while the detector observes all frequencies simultaneously.
An FTIR instrument can achieve the same signal-to-noise (S/N) ratio of a dispersive spectrometer in a fraction of the time (1 sec or less versus 10 to 15 min). The S/N ratio is proportional to the square root of the total number of measurements. Because multiple spectra can be readily collected in 1 min or less, sensitivity can be greatly improved by increasing S/N through co-addition of many repeated scans.
i. Increased optical throughput (Jaquinot advantage):
Energy-wasting slits are not required in the interferometer because dispersion or filtering is not needed. Instead, a circular optical aperture is commonly used in FTIR systems. The beam area of an FT instrument is usually 75 to 100 times larger than the slit width of a dispersive spectrometer. Thus, more radiation energy is made available. This constitutes a major advantage for many samples or sampling techniques that are energy-limited.
ii. Internal laser reference (Cannes advantage):
The use of a helium neon laser as the internal reference in many FTIR systems provides an automatic calibration in an accuracy of better than 0.01 cm-1. This eliminates the need for external calibrations.
iii. Simpler mechanical design:
There is only one moving part, the moving mirror, resulting in less wear and better reliability.
iv. Elimination of stray light and emission contributions:
The interferometer in FTIR modulates all the frequencies. The un-modulated stray light and sample emissions (if any) are not detected.
v. Powerful data station:
Modern FTIR spectrometers are usually equipped with a powerful, computerized data system. It can perform a wide variety of data processing tasks such as Fourier transformation, interactive spectral subtraction, baseline correction, smoothing, integration, and library searching.
Although the spectra of many samples can be satisfactorily run on either FTIR or dispersive instruments, FTIR spectrometers are the preferred choice tor samples that are energy-limited or when increased sensitivity is desired. A wide range of sampling accessories is available to take advantage of the capabilities of FTIR instruments.
Two-dimensional Infrared Spectroscopy:
Two-dimensional infrared correlation spectroscopy analysis is the application of 2D correlation analysis on infrared spectra. By extending the spectral information of a perturbed sample, spectral analysis is simplified and resolution is enhanced. The 2D synchronous and 2D asynchronous spectra represent a graphical overview of the spectral changes due to a perturbation (such as a changing concentration or changing temperature) as well as the relationship between the spectral changes at two different wave numbers.
Nonlinear Two-dimensional Infrared Spectroscopy:
It is the infrared version of correlation spectroscopy. Nonlinear two-dimensional infrared spectroscopy is a technique that has come available with the development of femtosecond infrared laser pulses. In this experiment first a set of pump pulses are applied to the sample. This is followed by a waiting time, where the system is allowed to relax.
The waiting time typically lasts from zero to several picoseconds and the duration can be controlled with a resolution of tens of femtoseconds. A probe pulse is then applied resulting in the emission of a signal from the sample. The nonlinear two-dimensional infrared spectrum is a two-dimensional correlation plot of the frequency ω1 that was excited by the initial pump pulses and the frequency ω3 excited by the probe pulse after the waiting time.
This allows the observation of coupling between different vibrational modes. Because of its extremely high time resolution it can be used to follow changes in molecular configurations taking place on a picosecond timescale. It is still a largely unexplored technique and is becoming increasingly popular for fundamental research.
Like in two-dimensional nuclear magnetic resonance (2DNMR) spectroscopy this technique spreads the spectrum in two dimensions and allow for the observation of cross peaks that contain information on the coupling between different modes. In contrast to 2DNMR nonlinear two dimensional infrared spectroscopy also involves the excitation to overtones.
These excitations result in excited state absorption peaks located below the diagonal and cross peaks. In 2DNMR two distinct techniques, COSY and NOESY are frequently used. The cross peaks in the first are related to the scalar coupling, while in the latter they are related to the spin transfer between different nuclei. In nonlinear two-dimensional infrared spectroscopy analogs have been drawn to these 2DNMR techniques.
Nonlinear two-dimensional infrared spectroscopy with zero waiting time corresponds to COSY and nonlinear two-dimensional infrared spectroscopy with finite waiting time allowing vibrational population transfer corresponds to NOESY. The COSY variant of nonlinear two dimensional infrared spectroscopy has been used for determination of the secondary structure cc intent proteins.
Uses and Applications:
Infrared spectroscopy is widely used in both research and industry as a simple and reliable technique tor measurement, quality control and dynamic measurement. The instruments are now small, and can be transported, even for use in field trials. With increasing technology in computer filtering and manipulation of the results, samples in solution can now be measured accurately (water produces a broad absorbance across the range of interest, and thus renders the spectra unreadable without this computer treatment). Some machines will also automatically tell you what substance is being measured from a store of thousands of reference spectra held in storage.
By measuring at a specific frequency over time, changes in the character or quantity of a particular bond can be measured. This is especially useful in measuring the degree of polymerization in polymer manufacture. Modern research machines can take infrared measurements across the whole range of interest as frequently as 32 times a second. This can be done whilst simultaneous measurements are made using other techniques. This makes the observations of chemical reactions and processes quicker and more accurate.
Techniques have been developed to assess the quality of tea-leaves using infrared spectroscopy. This will mean that highly trained experts (also called ‘noses’) can be used more sparingly, at a significant cost saving. Infrared spectroscopy has been highly successful for applications in both organic and inorganic chemistry.
Infrared spectroscopy has also been successfully utilized in the field of semiconductor microelectronics for example; infrared spectroscopy can be applied to semiconductors like silicon, gallium arsenide, gallium nitride, zinc selenide, amorphous silicon, silicon nitride, etc.
Near-Infrared Absorption Spectroscopy (NIR):
NIR spectroscopy is the measurement of the wavelength and intensity of the absorption of near- infrared light by a sample. Near-infrared light spans the 800 nm – 2.5 m (12500 – 4000 cm-1) range and is energetic enough to excite overtones and combinations of molecular vibrations to higher energy levels. NIR spectroscopy is typically used for quantitative measurement of organic functional groups, especially O—H, N—H, and C=Q Detection limits are typically 0.1% and applications include pharmaceutical, agricultural, polymer, and clinical analysis.
The components and design of NIR instrumentation are similar to UV-Vis absorption spectrometers. The light source is usually a tungsten lamp and the detector is usually a PbS solid-state detector. Sample holders can be glass or quartz and typical solvents are CCl4 and CS2. The convenient instrumentation of NIR spectroscopy compared to IR spectroscopy makes it much more suitable for on-line monitoring and process control.
Type # 8. Raman Spectroscopy:
Raman spectroscopy is a spectroscopic technique used in condensed matter physics and chemistry to study vibrational, rotational, and other low-frequency modes in a system. It relies on inelastic scattering, or Raman scattering of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range.
The laser light interacts with phonons or other excitations in the system, resulting in the energy of the laser photons being shifted up or down. The shift in energy gives information about the phonon modes in the system. Infrared spectroscopy yields similar, but complementary information. Typically, a sample is illuminated with a laser beam.
Light from the illuminated spot is collected with a lens and sent through a monochromator. Wavelengths close to the laser line (due to elastic Rayleigh scattering) are filtered out and those in a certain spectral window away from the laser line are dispersed onto a detector. Spontaneous Raman scattering is typically very weak, and as a result the main difficulty of Raman spectroscopy is separating the weak in elastically scattered light from the intense Rayleigh scattered laser light.
Raman spectrometers typically use holographic diffraction gratings and multiple dispersion stages to achieve a high degree of laser rejection. In the past, PMTs were the detectors of choice for dispersive Raman setups, which resulted in long acquisition times. However, the recent uses of CCD detectors have made dispersive Raman spectral acquisition much more rapid. Raman spectroscopy has a stimulated version, analogous to stimulated emission, called stimulated Raman scattering.
Basics:
The Raman Effect occurs when light impinges upon a molecule and interacts with the electron cloud of the bonds of that molecule. The incident photon excites one of the electrons into a virtual state. For the spontaneous Raman Effect, the molecule will be excited from the ground state to a virtual energy state, and relax into a vibrational excited state, which generates Stokes Raman scattering.
If the molecule was already in an elevated vibrational energy state, the Raman scattering is then called anti Stokes Raman scattering. A molecular polarizability change or amount of deformation of tin electron cloud, with respect to the vibrational coordinate is required for the molecule to exhibit the Raman Effect. The amount of the polarizability change will determine the intensity, whereas tin Raman shift is equal to the vibrational level that is involved.
History:
Although the inelastic scattering of light was predicted by Smekal in 1923, it was 1928 before it was observed in practice. The Raman Effect was named after one of its discoverers, the Indian scientist Sir C. V. Raman who observed the effect by means of sunlight (1928, together with K. S. Krishnan and independently by Grigory Landsberg and Leonid Mandelstam).
Raman won the Nobel Prize in Physics in 1930 for this discovery accomplished using sunlight, a narrow band photographic filter to create monochromatic light and a “crossed” filter to block this monochromatic light. He found that light of changed frequency passed through the “crossed” filter. Subsequently the mercury arc became the principal light source, first with photographic detection and then with spectrophoto metric detection. Currently lasers are used as light sources.
Applications:
Raman spectroscopy is commonly used in chemistry, since vibrational information is very specific for the chemical bonds in molecules. It, therefore, provides a fingerprint by which the molecule can be identified. The fingerprint region of organic molecules is in the range 500-2000 cm-1. Another way that the technique is used is to study changes in chemical bonding, e.g., when a substrate is added to an enzyme.
Raman gas analyzers have many practical applications, for instance they are used in medicine for real-time monitoring of anaesthetic and respiratory gas mixtures during surgery. In solid state physics, spontaneous Raman spectroscopy is used, among other things, to characterize materials, measure temperature, and find the crystallographic orientation of a sample.
As with single molecules, a given solid material has characteristic phonon modes that can help an experimenter identify it. In addition, Raman spectroscopy can be used to observe other low frequency excitations of the solid, such as plasmons, magnons, and superconducting gap excitations. The spontaneous Raman signal gives information on the population of a given phonon mode in the ratio between the Stokes (downshifted) intensity and anti-Stokes (upshifted) intensity.
Raman scattering by an anisotropic crystal gives information on the crystal orientation. The polarization of the Raman scattered light with respect to the crystal and the polarization of the laser light can be used to find the orientation of the crystal, it the crystal structure (specifically, its point group) is known. Raman active fibres, such as aramid and carbon, have vibrational modes that show a shift in Raman frequency with applied stress.
Polypropylene fibres also exhibit similar shifts. The radial breathing mode is a commonly used technique to evaluate the diameter of carbon nanotubes. Spatially Offset Raman Spectroscopy (SORS), which is less sensitive to surface layers than conventional Raman, can be used to discover counterfeit drugs without opening their internal packaging, and for non-invasive monitoring of biological tissue.
Raman spectroscopy can be used to investigate the chemical composition of historical documents such as the Book of Kells and contribute to knowledge of the social and economic conditions at the time the documents were produced. This is especially helpful because Raman spectroscopy offers a non-invasive way to determine the best course of preservation or conservation treatment for such materials.
Raman Micro Spectroscopy:
Raman spectroscopy offers several advantages for microscopic analysis. Since it is a scattering technique, specimens do not need to be fixed or sectioned. Raman spectra can be collected from a very small volume (< 1 µm in diameter); these spectra allow the identification of species present in that volume. Water does not interfere very strongly. Thus, Raman spectroscopy is suitable for the microscopic examination of minerals, materials such as polymers and ceramics, cells and proteins.
A Raman microscope begins with a standard optical microscope, and adds an excitation laser, a monochromator, and a sensitive detector (such as a charge-coupled device (CCD) or photomultiplier tube (PMT)). FT-Raman has also been used with microscopes. In direct imaging, the whole field of view is examined tor scattering over a small range of wave numbers (Raman shifts). For instance, a wave number characteristic for cholesterol could be used to record the distribution of cholesterol within a cell culture. The other approach is hyper spectral imaging or chemical imaging, in which thousands of Raman spectra are acquired from all over the field of view.
The data can then be used to generate images showing the location and amount of different components. Taking the cell culture example, a hyper spectral image could show the distribution of cholesterol, as well as proteins, nucleic acids, and fatty acids. Sophisticated signal- and image-processing techniques can be used to ignore the presence of water, culture media, buffers, and other interferences.
Raman microscopy, and in particular confocal microscopy, has very high spatial resolution. For example, the lateral and depth resolutions were 250 nm and 1.7 µm, respectively, using a confocal Raman micro spectrometer with the 632.8 nm line from a He-Ne laser with a pinhole of 100 diameters.
Since the objective lenses of microscopes focus the laser beam to several micro-metres in diameter, the resulting photon flux is much higher than achieved in conventional Raman set-ups. This has the added benefit of enhanced fluorescence quenching. However, the high photon flux can also cause sample degradation, and for this reason some setups require a thermally conducting substrate (which acts as a heat sink) in order to mitigate this process.
By using Raman micro spectroscopy, in vivo time- and space-resolved Raman spectra of microscopic regions of samples can be measured. As a result, the fluorescence of water, media, and buffers can be removed. Consequently in vivo time- and space-resolved Raman spectroscopy is suitable to examine proteins, cells and organs. Raman microscopy for biological and medical specimens generally uses near-infrared (NIR) lasers (785 nm diodes and 1064 nm Nd:YAG an especially common).
This reduces the risk of damaging the specimen by applying high power. However, the intensity of NIR Raman is low (owing to the ω-4 dependence of Raman scattering intensity), and most detectors required very long collection times. Recently, more sensitive detectors have become available, making the technique better suited to general use. Raman microscopy of inorganic specimens, such as rocks and ceramics and polymers, can use a broader range of excitation wavelengths.
Variations:
Several variations of Raman spectroscopy have been developed. The usual purpose is to enhance the sensitivity (e.g., surface-enhanced Raman), to improve the spatial resolution (Raman microscopy}, or to acquire very specific information (resonance Raman).
1. Surface Enhanced Raman Spectroscopy (SERS):
Normally done in a silver or gold colloid or a substrate containing silver or gold. Surface Plasmon’s of silver and gold are easily excited by the laser, and the resulting electric fields cause other nearby molecules to become Raman active. The result is amplification of the Raman signal (by up to 1011). This effect was originally observed by Fleishman but the prevailing explanation was proposed by Van Duyne in 1977.
2. Hyper Raman:
A non-linear effect in which the vibrational modes interact with the second harmonic of the excitation beam. This requires very high power, but allows the observation of vibrational modes which are normally “silent”. It frequently relies on SERS-type enhancement to boost the sensitivity.
3. Resonance Raman Spectroscopy:
The excitation wavelength is matched to an electronic transition of the molecule or crystal, so that vibrational modes associated with the excited electronic state are greatly enhanced. This is useful for studying large molecules such as polypeptides, which might show hundreds of bands in “conventional” Raman spectra. It is also useful for associating normal modes with their observed frequency shifts.
4. Spontaneous Raman Spectroscopy:
Used to study the temperature dependence of the Raman spectra of molecules.
5. Optical Tweezers Raman Spectroscopy (OTRS):
Used to study individual particles, and even biochemical processes in single cells trapped by optical tweezers.
6. Stimulated Raman Spectroscopy:
A two-colour pulse transfers the population from ground to a vibrationally excited state, if the difference in energy corresponds to an allowed Raman transition. Two-photon UV ionization, applied after the population transfer but before relaxation, allows the intra-molecular or inter-molecular Raman spectrum of a gas or molecular cluster (indeed, a given conformation of molecular cluster) to be collected. This is a useful molecular dynamics technique.
7. Spatially Offset Raman Spectroscopy (SORS):
The Raman scatter is collected from regions laterally offset away from the excitation laser spot, leading to significantly lower contributions from the surface layer than with traditional Raman spectroscopy.
8. Coherent Anti-Stokes Raman Spectroscopy (CARS):
Two laser beams are used to generate a coherent anti-Stokes frequency beam, which can be enhanced by resonance.
9. Resonance Enhanced Multi-Photon Ionization (REMPI):
Is a technique applied to the spectroscopy of atoms and small molecules. In practice, a tunable laser can be used to access an excited intermediate state. The selection rules associated with a two-photon or other multi-photon photo absorption are different from the selection rules for a single photon transition.
The REMPI technique typically involves a resonant single or multiple photon absorption to an electronically excited intermediate state followed by another photon which ionizes the atom or molecule. The light intensity to achieve a typical multi-photon transition is generally significantly larger than the light intensity to achieve a single photon photo absorption.
Because of this, a subsequent photo-absorption is often very likely. An ion and a free electron will result if the photons have imparted enough energy to exceed the ionization threshold energy of the system. In many cases, REMPI provides spectroscopic information that can be unavailable to single photon in spectroscopic methods, for example rotational structure in molecules is easily seen with this technique.
Rydberg States, Rydberg Molecules:
High photon intensity experiments can involve multi-photon processes with the absorption of integer multiples of the photon energy. In experiments that involve a multi-photon resonance, the intermediate is often a low-lying Rydberg state, and the final state is often an ion. The initial state of the system, photon energy, angular momentum and other selection rules can help in determining the nature of the intermediate state.
This approach is exploited in Resonance Enhanced Multi-Photon Ionization Spectroscopy (REMPI). The technique is in wide use in both atomic and molecular spectroscopy. An advantage of the REM PI technique is that the ions can be detected with almost complete efficiency and even time resolved for their mass.
It is also possible to gain additional information by performing experiments to look at the energy of the liberated photoelectron in these experiments. (Robert N. Compton formerly of O.R.N.L. and Philip M. Johnson pioneered the development of REMPI. The technique was named by Johnson.)
Type # 9. Mass Spectroscopy:
Mass Spectrometry is one of the most powerful analytical tools that the chemist has for the characterisation of individual compounds. The principles of the technique have been established for some time although several new methods of ionisation have been developed recently.
Basics:
All mass spectrometers require the sample compound to be ionised and those ions to be transferred into the gas phase for analysis; this may be a simultaneous process or a distinct series of events. In conventional Electron Impact Ionisation (EI], the sample is vaporized and then ionised by collisions with a beam of energetic electrons.
The ions produced are accelerated by an applied electric field and then they are separated according to their mass to charge ratio either as a continuous beam or a discrete pulse. After separation the ions arrive at a detector and produce a small electric current, which is amplified and recorded by a computer-controlled system.
The Spectrum:
Most ions formed by conventional techniques are singly charged. The mass spectrum is produced by relating a scan of the analyser to the signals received at the detector. In the cast of a magnetic sector instrument, the signal is measured as a function of magnetic field strength.
The magnetic field strength can be related to the ion mass, thus yielding a plot of signal intensity versus mass the ma spectrum. From the spectrum it is often possible to determine the molecular mass of the compound, some information about its structure and, if in a mixture, its concentration.
The Ion Source:
Some instruments have a single means of sample introduction and ionisation, others may have many. EI requires the sample to be vaporized and, therefore, solids are introduced on a heatable probe, liquids via a heated septum and bleed valve, and gases through a membrane or a needle valve system. The vapour is crossed by an energetic electron beam, which can ionise the sample giving molecular ions, as well as fragment ions, all with a positive charge.
FAB [fast atom bombardment] requires samples to be dissolved in a matrix such as glycerol and, therefore, the sample must be inserted on a probe system. The surface of this mixture is subjected to a beam of energetic xenon [or argon] atoms which sputter ions from the mixture. These may be positive or negative and each can be detected separately.
ESI [i.e., electrospray] is a technique that ionizes directly from the liquid state and at atmospheric pressure. The idea is simple and relies on the solution being ionic. The ionic solution is pumped through a capillary at high electrical potential and the ions are rapidly separated from solvent molecules and their corresponding counter-ions. The ions then pass through a tiny nozzle connected to the vacuum system and enter the spectrometer. Again, ions formed in this manner may be positive or negative depending on the applied potential.
MALDI [matrix assisted laser desorption ionisation] works on solid sample spots co deposited with a light-absorbing matrix, e.g., cyanohydroxycinnamic acid. By irradiating these sample spots in the matrix with high intensity laser radiation, both positive and negative ions, are produced.
The Analyser:
Magnetic Sectors are the traditional means of separating ions of different masses using the principle that when a beam of ions passes through a magnetic field they are deflected through an arc whose radius is related to the ratio of mass to charge. By varying the field in a known manner, i.e., sweeping from high to low field with time, ions of different mass arrive at the detector sequentially and produce the mass spectrum.
Quadrupoles, often used on smaller instruments linked to chromatographic systems, use an oscillating electric field between opposite pairs of a set of 4 rods. The beam of ions passing between these rods is deflected by differing amounts depending on their mass/charge ratio. By varying the held strength or the frequency of oscillation, ions of different mass reach the detector to produce a mass spectrum.
Time-of-Flight — Given the same amount of energy [e.g., 20 kV potential] ions of different mass will take different times to travel the same distance, the lightest being the fastest. This idea is put to use in time-of-flight instruments.
In essence, these consist of an ion source/acceleration region, a drift tube along which the ions travel, and finally a detector which measures the ion signal at the end of the drift tube as a function of time. The short pulse time of laser ionisation using intense laser pulses is ideally suited to this form of analyser.
Other types of analysers, e.g., ion traps, cyclotron resonance, are also used in mass spectrometry but are beyond the scope of these brief notes.
Detectors:
These are usually electron multipliers which produce up to 106 electrons for every ion that hits the detectors. This produces a current which can be amplified and measured.
The Mass Spectrum:
The example below illustrates a typical EI spectrum:
[C8H7OBr – Ethanone, 2-bromo-l-phenyl-]
Ions are shown as vertical lines of differing intensity along an x-axis of mass/charge. As all the charges here are ‘1+’ the scale can be regarded as that of mass. Various features of the spectrum may be distinguished, e.g., the ions at highest mass [in this case a small cluster at 198/200]. These un-fragmented ions are called the ‘molecular ions’ (sometimes also known as parent ions) with fragment ions at 120, 105, 91, 77, etc.
These fragment ions arise by the loss of certain group’s clue to the high energy of electron impact. The fragments may be easily identified as belonging to a specific group and can be used as a fingerprint for the molecule if produced under standard conditions. A mass of 77 is usually C6H+5, 91 is C7H+7, etc. The molecular ion at 198/200 is also very informative.
The fact that it comprises two main peaks separated by 2 mass units and of similar intensities indicates that the molecule contains bromine [2 isotopes 79/81]; also as the mass is even this indicates that the molecule contains an oxygen atom or an even number of nitrogen atoms.
In this case 105 is probably [M minus (Br-CH2)] +, i.e., 198-93.
As a contrast the ESI spectrum below is of a protein:
The mass of this protein is 16951 but because of the multiple charges on the ions formed under electrospray conditions the various ion clusters appear as a pattern on the normal scale of 800-2000. The separation of the various ions allows the allocation of the number of charges by application of a suitable algorithm; hence the numbers in this case show ions of +10 to +21; this in turn [assuming that the ions are formed by association with H+] allows the calculation of the true molecular mass of the protein. Very few fragment ions are observed, although impurities may contribute to some of the extra signals.
Instrumental Considerations:
(a) Resolution:
The resolution of any mass spectrometer determines its ability to distinguish between adjacent masses; for many applications it is only required to separate unit values of mass, e.g., 500 from 501, but where there is the possibility of mass differences of 0.01 or smaller the ability to increase the resolution is necessary.
Quadrupoles generally only work at unit mass differences [the resolution defined as R = m/(m1-m2), where m is the average of the ions and m1-m2 the mass difference, with peak separation showing a valley between the ions of 50% peak height]. Two-Sector instruments usually have the ability to adjust the resolution and can resolve peaks 500.00 and 500.01 to a valley of 10% peak height or better. Resolution in time-of-flight instruments is somewhere between these other two instrument types.
(b) Mass accuracy:
The ability to measure masses to a high degree of accuracy is required tor the determination of the elemental composition of a molecule; generally this is only achieved on two-sector instruments (and some of the more specialised techniques, e.g., Fourier Transform Ion Cyclotron Resonance). The accuracy depends on many factors, but crucial is the use of accurately known calibrant ion peaks.
(c) Sensitivity:
Mass Spectrometers are very sensitive, often operating with very minute amounts of material, e.g., 1 femtogram (0.000000000000001g). However, different ionisation techniques have different efficiencies and so EI and MALDI are the most sensitive with ESI close and FAB the least sensitive.
Uses of Mass Spectrometry:
Chemical Identification — by means of fragmentation and accurate mass.
Forensic Testing — drugs, explosives, etc. are often detected using mass spectrometry.
Environmental Monitoring— atmospheric pollutants, spillage incidents and various emissions can be effectively detected and mapped.
Pharmaceutical Testing — new drugs, etc., have to be thoroughly tested for side effects and harmful metabolites before release for use. Mass spectrometry is one of the main analytical techniques used.
Archaeological Studies — isotope ratios are measured by mass spectrometry and used to determine the age of artefacts and often the origin of materials.
Medical Research — Many illnesses produce particular chemical species in the body, or changes to normal components. These may be detected and monitored by suitable mass spectrometry techniques.
Biological Studies — The natural world produces many active chemical species that may be useful, their detection and formation pathways may often require mass spectrometry.
Type # 10. Electron Paramagnetic Resonance:
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a technique tor studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion. The basic physical concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but it is electron spins that are excited instead of spins of atomic nuclei.
Since most stable molecules have all their electrons paired, the EPR technique is less widely used than NMR. However, this limitation to paramagnetic species also means that the EPR technique is one of great specificity, since ordinary chemical solvents and matrices do not give rise to EPR spectra.
Origin of an EPR Signal:
Every electron has a magnetic moment and spin quantum number s = 1/2, with magnetic components ms = +1/2 and ms = —1/2. In the presence of an external magnetic field with strength B0, the electron’s magnetic moment aligns itself either parallel (ms = -1/2) or antiparallel (m + 1/2) to the field, each alignment having a specific energy.
The parallel alignment corresponds to the lower energy state, and the separation between it and the upper state is ∆E = geµBB0, where ge is the electron’s so-called g-factor. This equation implies that the splitting of the energy levels is directly proportional to the magnetic field’s strength, as shown in the diagram below.
An unpaired electron can move between the two energy levels by either absorbing or emitting electromagnetic radiation of energy ɛ = hv such that the resonance condition, ɛ = ∆E, is obeyed. Substituting in ɛ = hv and ∆E = geµBB0 leads to the fundamental equation of EPR spectroscopy: hv – geµBB0.
Experimentally, this equation permits a large combination of frequency and magnetic field values, but the great majority of EPR measurements are made with microwaves in the 9000 – 10000 MHz (9 10 GHz) region, with fields corresponding to about 3500 G (0.3500 T). In principle, EPR spectra can be generated by either varying the photon frequency incident on a sample while holding the magnetic field constant, or doing the reverse.
In practice, it is usually the frequency which is kept fixed. A collection of paramagnetic centres, such as free radicals, is exposed to microwaves at a fixed frequency. By increasing an external magnetic field, the gap between the ms = +1/2 and ms = —1/2 energy states is widened until it matches the energy of the microwaves, as represented by the double-arrow in the diagram above.
At this point the unpaired electrons can move between their two spin states. Since there are typically more electrons in the lower state, due to the Maxwell-Boltzmann distribution, there is a net absorption of energy, and it is this absorption which is monitored and converted into a spectrum.
As an example of how hv = geµBB0 can be used, consider the case of a free electron, which has ge = 2.0023, and the simulated spectrum shown at the right in two different forms. For the microwave frequency of 9388.2 MHz, the predicted resonance position is a magnetic field of about B0 hv/ geµB = 0.3350 tesla = 3350 gauss, as shown.
Note that while two forms of the same spectrum are presented in the figure, most EPR spectra are recorded and published only as first derivatives. Because of electron-nuclear mass differences, the magnetic moment of an electron is substantially larger than the corresponding quantity for any nucleus, so that a much higher electromagnetic frequency is needed to bring about a spin resonance with an electron than with a nucleus, at identical magnetic field strengths.
For example, for the field of 3350 G shown at the right, spin resonance occurs near 9388.2 MHz for an electron compared to only about 14.3 MHz for 1H nuclei. (For NMR spectroscopy, the corresponding resonance equation is hv – gNµNB0, where gN and µN depend on the nucleus under study.)
Maxwell-Boltzmann Distribution:
In practice, EPR samples consist of collections of many paramagnetic species, and not single isolated paramagnetic centers. If the population of radicals is in thermodynamic equilibrium, its statistical distribution is described by the Maxwell-Boltzman equation
where nupper is the number of paramagnetic centres occupying the upper energy state, k is the Boltzmann constant, and T is the temperature in kelvin. At 298 K, X-band microwave frequencies (v ≈ 9.75 GHz) give nupper / nlower ≈ 0.998, meaning that the upper energy level has a mailer population than the lower one.
Therefore, transitions from the lower to the higher level are more probable than the reverse, which is why there is a net absorption of energy. The sensitivity of the EPR method (i.e., the minimum number of detectable spins, Nmin) depends on the photon frequency v according to
Nmin = k1V/Q0kfv2P1/2,
where k1 is a constant, V is the sample’s volume, Q0 is the unloaded quality factor of the microwave cavity (sample chamber), kf is the cavity filling coefficient, and P is the microwave power in the spectrometer cavity. With kf and P being constants, Nmin ~ (Q0V2)-1, i.e., Nmin ~ v-α, where α ≈ 1.5. In practice, α can change varying from 0.5 to 4.5 depending on spectrometer characteristics, resonance conditions, and sample size. In other words, the higher the spectrometer frequency the lower the detection limit (Nmin), meaning greater sensitivity.
EPR Spectral Parameters:
In real systems, electrons are normally not solitary, but are associated with one or more atoms.
There are several important consequences of this:
1. An unpaired electron can gain or lose angular momentum, which can change the value of its g-factor, causing it to differ from ge. This is especially significant for chemical systems with transition-metal ions.
2. It an atom with which an unpaired electron is associated has a non-zero nuclear spin, and then its magnetic moment will affect the electron. This leads to the phenomenon of hyperfine coupling, analogous to J-coupling in NMR, splitting the EPR resonance signal into doublets, triplets and so forth.
3. Interactions of an unpaired electron with its environment influence the shape of an EPR spectral line. Line shapes can yield information about, for example, rates of chemical reactions.
4. The g-factor and hyperfine coupling in an atom or molecule may not be the same for all orientations of an unparied electron in an external magnetic field. This anisotropy tie pends upon the electronic structure of the atom or molecule (e.g., free radical) in question, and so can provide information about the atomic or molecular orbital containing the unpaired electron.
The g-factor:
Knowledge of the g-factor can give information about a paramagnetic centre’s electronic structure. An unpaired electron responds not only to a spectrometer’s applied magnetic field B0, but also to any local magnetic fields of atoms or molecules. The effective field Beff experienced by an electron is thus written as,
Beff = B0(1 – σ),
where σ includes the effects of local fields (σ can be positive or negative).Therefore, the hv = geµBBeff resonance condition (above) is rewritten as follows,
hv = geµBBeff = geµBB0(1 – σ).
The quantity ge( 1 — σ) is denoted by g and called simply the g-factor, so that the final resonance equation becomes
hv = gµBB0.
This last equation is used to determine g in an EPR experiment by measuring the field and the frequency at which resonance occurs. If g does not equal ge the implication is that the electron’s spin magnetic moment is constant (approximately the Bohr magneton), then the electron must have gained or lost angular momentum through spin-orbit coupling. Because the mechanisms of spin orbit coupling are well understood, the magnitude of the change gives information about the nature of the atomic or molecular orbital containing the unpaired electron.
Since the source of an EPR spectrum is a change in an electron’s spin state, it might be thought that all EPR spectra would consist of a single line. However, the intereaction of an unpaired electron, by way of its magnetic moment, with nearby nuclear spins, results in additional allowed energy states and, in turn, multi-lined spectra.
In such cases, the spacing between the EPR spectral lines indicates the degree of interaction between the unpaired electron and the perturbing nuclei. Two common mechanisms by which electrons and spectral line spacing and, in the simplest cases, is essentially the spacing itself.
Two common mechanisms by which electrons and nuclei interact are Fermi-contact interaction and dipolar interaction. The former applies largely to the case of isotropic interactions (independent of sample orientation in a magnetic field) and the latter to the case of anisotropic interactions (spectra dependent on sample orientation in a magnetic field).
Spin polarization is a third mechanism for interactions between an unpaired electron and a nuclear spin, being especially important for π-electron organic radicals, such as the benzene radical anion. The symbols “a” or “A” are used for isotropic hyperfine coupling constants while “B” is usually employed tor anisotropic hyperfine coupling constants. In many cases like some explained below, the isotropic hyperfine splitting pattern tor a radical freely tumbling in a solution (isotropic system) can be predicted.
i. For a radical having M equivalent nuclei, each with a spin of I, the number of FPR lines expected is 2MI + 1. As an example, the methyl radical, CH3, has three 1H nuclei each with I = 1/2, and so the number of lines expected is 2MI + 1 = 2(3)(1/2) + 1—4, which is as observed.
ii. For a radical having M1 equivalent nuclei, each with a spin of I1, and a group of M2 equivalent nuclei, each with a spin of I2, the number of lines expected is (2M1I1 + 1)(2M2I2 + 1). As an example, the methoxvmethyl radical, H2C(OCH3), has two equivalent 1H nuclei each with I = 1/2 and three equivalent 1H nuclei each with I = 1/2, and so the number of lines expected is (2M1I1 + 1)(2M2I2 + 1) = [2(2)(1/2) + 1] [2(3)(1/2) + 1] = [3] [4] = 12, again as observed.
iii. The above can be extended to predict the number of lines for any number of nuclei.
While it is easy to predict the number of lines a radical’s EPR spectrum should show, the reverse problem, unraveling a complex multi-line EPR spectrum and assigning the various spacing’s to specific nuclei, is more difficult. In the oft-encountered case of I = 1/2 nuclei (e.g., 1H, 19F, 31P), the line intensities produced by a population of radicals, each possessing M equivalent nuclei, will follow Pascal’s triangle.
For example, the spectrum at the right shows that the three 111 nuclei of the CH3 radical give rise to 2MI + 1 = 2(3)(1/2) + 1=4 lines with a 1:3:3:1 ratio. The line spacing gives a hyperfine coupling constant of aH = 23 G for each of the three 1H nuclei. Note again that the lines in this spectrum are first derivatives of absorptions.
As a second example, consider the methoxy-methyl radical, H2C (OCH3). The two equivalent methyl hydrogen’s will give an overall 1:2:1 EPR pattern, each component which s further split In the three methoxy hydrogen’s into a 1:3:3:1 pattern to give a total of 3 × 4= 12 lines, a triplet of quartets. A simulation of the observed EPR spectrum is shown at the right, and agrees with the 12-line prediction and the expected line intensities.
Note that the smaller coupling constant (smaller line spacing) is due to the three methoxy hydrogen’s, while the larger coupling constant (line spacing) is from the two hydrogen’s bonded directly to the carbon atom bearing the unpaired electron. It is often the case that coupling constants decrease in size with distance from a radical’s unpaired electron, but there are some notable exceptions, such as the ethyl radical (CH2CF3).
Resonance Linewidth Definition:
Resonance linewidths are defined in terms of the magnetic induction B, and its corresponding units and are measured along the x-axis of an EPR spectrum, from a line’s centre to a chosen reference point of the line. These defined widths are called half widths and possess some advantages; or asymmetric lines values of left and right half width can be given.
The half width ∆Bh is the distance measured from the line’s centre to the point in which absorption value has half of maximal absorption value in the centre of resonance line. First inclination width ∆B1/2 is a distance from centre of the line to the point of maximal absorption curve inclination. In practice, a full definition of line width is used. For symmetric lines, half width ∆B1/2 = 2∆Bh, and full inclination width ∆Bmax = 2∆B1s.
The EPR waveband is stipulated by the frequency or wavelength of a spectrometer’s microwave source (see Table). EPR experiments often are conducted at X and, less commonly, Q bands, mainly due to the ready availability of the necessary microwave components (which originally were developed for radar applications).
A second reason for widespread X and Q band measurements is that electromagnets can reliably generate fields up to about 1 tesla. However, the low spectral resolution over g-factor at these wavebands limits the study of paramagnetic centres with comparatively low anisotropic magnetic parameters.
Measurements at V > 40 GHz, in the millimetre wavelength region, offer the following advantages:
(a) EPR spectra are simplified due to the reduction of second-order effects at high fields.
(b) Increase in orientation selectivity and sensitivity in the investigation of disordered systems.
(c) The informatively and precision of pulse methods, e.g., KNDOR also increase at high magnetic fields.
(d) Accessibility of spin systems with larger zero-field splitting due to the larger microwave quantum energy hv.
(e) The higher spectral resolution over g factor, which increases with irradiation frequency v and external magnetic field B0. This is used to investigate the structure, polarity, and dynamics of radical microenvironments in spin-modified organic and biological systems through the spin label and probe method. The figure shows how spectral resolution improves with increasing frequency.
(f) Saturation of paramagnetic centres occurs at a comparatively low microwave polarizing field B1, due to the exponential dependence of the number of excited spins on the radiation frequency V. This effect can be successfully used to study the relaxation and dynamics of paramagnetic centres as well as of super slow motion in the systems under study. The cross-relaxation of paramagnetic centres decreases dramatically at high magnetic fields, making it easier to obtain more-precise and more-complete information about the system under study.
EPR Applications:
EPR is used in solid-state physics, for the identification and quantification of radicals in chemistry to identify reaction pathways, as well as in biology and medicine for tagging biological spin probes. Since radicals are very reactive, they do not normally occur in high concentrations in biological environments. With the help of specially designed nonreactive radical molecules that attach to specific sites in a biological cell, it is possible to obtain information on the environment of these so- called spin label or spin-probe molecules.
Type # 11. NMR Spectroscopy:
Nuclear magnetic resonance spectroscopy most commonly known as NMR spectroscopy is filename given to the technique which exploits the magnetic properties of certain nuclei. This phenomenon and its origins are detailed in a separate section on Nuclear magnetic resonance. The most important applications for the organic chemist are proton NMR and carbon-13 NMR, although in principle NMR is applicable to any nucleus possessing spin.
Many types of information can be obtained from this single phenomenon. Much like using infrared spectroscopy to identity functional groups, analysis of a ID NMR spectrum tells the scientist what chemical environments are present and (in most cases) their relative abundances within the sample. The impact of NMR spectroscopy on the natural sciences has been substantial.
It can, amongst other things, be used to study mixture of analytes, to understand dynamic effects such as change in temperature and reaction mechanisms, and is an invaluable tool in understanding protein and nucleic acid structure and function. It can be applied to a wide variety- of samples, both in the solution and the solid state.
Basic NMR Techniques:
When placed in a magnetic field, NMR active nuclei (like 1H or 13C) resonate at a specific frequency for each element or isotope directly proportional to the strength of the magnetic field, For example, in a 21 tesla magnetic field, proton- resonate at 900 MHz It is common to refer to a 21 T magnet as a 900 MHz magnet but different nuclei resonate at a different frequent at this field strength.
Chemical Shift:
Depending on the local chemical environment, different protons in a molecule resonate at slightly different frequencies. Since this frequency shift is proportional to the strength of the magnetic field, it is converted into a field-independent dimensionless value known as the chemical shift. The chemical shift is reported as a relative measure from some reference resonance frequency. (For the nuclei 1H, 13C, and 29Si, TMS (tetramethylsilane) is commonly used as a reference.)
This difference between the frequency of the signal and the frequency of the reference is divided by frequency of the reference signal to give the chemical shift. The frequency shifts are extremely small (say 100s of Hz) in comparison to the fundamental NMR frequency (typically 100s of MHz) and so are generally expressed as parts per million (ppm).
By understanding different chemical environments, the chemical shift can be used to obtain some structural information about the molecule in question to assign signals to an atom or a group of atoms. For example, in a proton spectrum for ethanol (CH3CH2OH) one would expect three specific signals at three specific chemical shifts.
One for the CH3 group, one for the CH2 group and one for the OH. A typical CH3 group has a shift around 1 ppm, the CH2 attached to a OH has a shift of around 4 ppm and the OH has a shift around 2-3 ppm depending on the solvent used. Because the molecular motion at room temperature makes each of the three methyl protons average out during the course of the NMR experiment (which typically takes a few ms), the proton become degenerate and form a peak at the same chemical shift.
Interestingly the- shape and size of peaks are indicators of chemical structure too. In the example above—the proton spectrum of ethanol—the CH3 peak would be three times as large as the OH. Similarly, the CH2 peak would be twice the size of the OH peak but only 2/3 of the size of the CH3 peak. Modern analysis software allows analysis of the size of peaks to understand how many protons give rise to the peak.
This is known as integration—a mathematical process which calculates the area under a graph (essentially what a spectrum is). The analyst must integrate the peak and not measure its height because the peaks also have width, and thus its size is dependent on its area not on its height. However, it should be mentioned that the number of protons, or any other observed nucleus, is only proportional to the intensity, or the integral of the NMR signal, in the very simplest one-dimensional NMR experiments.
In more elaborate experiments, for instance, experiments typically used to obtain carbon 13 NMR spectra; the integral of the signals depends on the relaxation rate of the nucleus, and its scalar and dipolar coupling constants. Very often these factors are poorly understood. Therefore, the integral of the NMR signal is very difficult to interpret in more complicated NMR experiments.
J-coupling:
Some of the most useful information for structure determination in a one dimensional NMR spectrum comes from J-coupling or scalar coupling (a special case of spin-spin coupling) between NMR active nuclei. This coupling arises from the interaction of different spin states through the chemical bonds of a molecule and results in the splitting of NMR signals into recognizable patterns based on the pairing of spin states.
As a result, this coupling can tell us information about the connectivity of atoms in a molecule. Coupling to n equivalent (spin ½) nuclei splits the signal into a n+1 multiplet with intensity ratios following Pascal’s triangle as described on the right. Coupling to additional spins will lead to further splitting’s of each component of the multiplet, e.g., coupling to two different spin ½ nuclei with significantly different coupling constants will lead to a doublet of doublets (abbreviation: dd).
Note that coupling between nuclei that are chemically equivalent (that is, have the same chemical shift) has no effect of the NMR spectra and couplings between nuclei that are distant (usually more than 3 bonds apart for protons in flexible molecules) are usually too small to cause observable splitting’s. Long-range couplings over more than three bonds can often be observed in cyclic and aromatic compounds, leading to more complex splitting patterns.
For example, in the proton spectrum for ethanol described above, the CH3 group is split into a triplet with an intensity ratio of 1:2:1 by the two neighbouring CH2 protons. Similarly, the CH2 is split into a quartet with an intensity ratio of 1:3:3:1 by the three neighbouring CH3 protons. In principle, the two CH2 protons would also be split again into a doublet to form a doublet of quartets by the hydroxyl proton, but intermolecular exchange of the acidic hydroxyl proton often results in a loss of coupling information.
Coupling to any spin ½ nuclei such as phosphorus-31 or fluorine-19 works in this fashion (although the magnitudes of the coupling constants may be very different). But the splitting patterns differ from those described above for nuclei with spin greater than ½ because the spin quantum number has more than two possible values. For instance, coupling to deuterium (a spin 1 nucleus) splits the signal into a 1:1:1 triplet because the spin 1 has three spin states.
Similarly, a spin 3/2 nucleus splits a signal into a 1:1:1:1 quartet and so on. Coupling combined with the chemical shift (and the integration for protons) tells us not only about the chemical environment of the nuclei, but also the number of neighbouring NMR active nuclei within the molecule. In more complex spectra with multiple peaks at similar chemical shifts or in spectra of nuclei other than hydrogen, coupling is often the only way to distinguish different nuclei.
Second-order (or strong) Coupling:
The above description assumes that the coupling constant is small in comparison with the difference in NMR frequencies between the in-equivalent spins. If the shift separation decreases (or the coupling strength increases), the multiplet intensity patterns are first distorted, and then become more complex and less easily analyzed (especially if more than two spins are involved).
Intensification of some peaks in a multiplet is achieved at the expense of the remainder, which sometimes almost disappear in the background noise, although the integrated area under the peaks remains constant In most high-field NMR, however, the distortions are usually modest and the characteristic distortions (roofing) can in fact help to identify related peaks.
Second-order effects decrease as the frequency difference between multiplets increases, so that high-field (i.e., high-frequency) NMR spectra display less distortion than lower frequency spectra. Early spectra at 60 MHz were more prone to distortion than spectra from later machines typically operating at frequencies at 200 MHz or above.
Magnetic In-equivalence:
More subtle effects can occur if chemically equivalent spins (i.e., nuclei related by symmetry and so having the same NMR frequency) have different coupling relationships to external spins. Spins that are chemically equivalent but are not indistinguishable (based on their coupling relationships) are termed magnetically in-equivalent.
For example, the 4 H sites of 1,2-dichlorobenzene divide into two chemically equivalent pairs by symmetry, but an individual member of one of the pairs has different couplings to the spins making up the other pair. Magnetic in-equivalence leads to highly complex spectra which can only be analyzed by computational modelling.
Such effects are more common in NMR spectra of aromatic and other non-flexible systems, while rapid rotation about C-C bonds in flexible molecules ensures that the couplings between protons on adjacent carbons are identical, avoiding problems with magnetic in-equivalence.
Correlation Spectroscopy:
Correlation spectroscopy is one of several types of two-dimensional nuclear magnetic resonance (NMR) spectroscopy. This type of NMR experiment is best known by its acronym, COSY. Other types of two-dimensional NMR include J-spectroscopy, exchange spectroscopy (FXSY), Nuclear Over Hauser effect spectroscopy (NOFSY), total correlation spectroscopy (TOCSY) and hetero-nuclear correlation experiments, such as HSQC, HMQC, and HMBC.
Two-dimensional NMR spectra provide more information about a molecule than one-dimensional NMR spectra and are especially useful in determining the structure of a molecule, particularly for molecules that are too complicated to work with using one-dimensional NMR.
The first two-dimensional experiment, COSY, was proposed by Jean jeener, a professor at University Libre de Bruxelles, in 1971 This experiment was later implemented by Walter P. Aue, Enrico Bartholdi and Richard R. Ernst, who published their work in 1976.
Solid-State Nuclear Magnetic Resonance:
A variety of physical circumstances does not allow molecules to be studied in solution and at the same time not by other spectroscopic techniques to an atomic level, either. In solid-phase media, such as crystals, microcrystalline powders, gels, anisotropic solutions, etc., it is in particular the dipolar coupling and chemical shift anisotropy that become dominant to the behaviour of the nuclear spin systems.
In conventional solution-state NMR spectroscopy, these additional interactions would lead to a significant broadening of spectral lines. A variety of techniques allows to establish high resolution conditions, that can, at least for 13C spectra, be comparable to solution-state NMR spectra. Two important concepts for high-resolution solid-state NMR spectroscopy are the limitation of possible molecular orientation by sample orientation, and the reduction of anisotropic nuclear magnetic interactions by sample spinning.
Of the latter approach, fast spinning around the magic angle is a yen prominent method, when the system comprises spin 1/2 nuclei. A number of intermediate techniques, with samples of partial alignment or reduced mobility, is currently being used in NMR spectroscopy. Applications in which solid-state NMR effects occur are often related to structure investigations on membrane proteins, protein fibrils or all kinds of polymers, and chemical analysis in inorganic chemistry, but also include “exotic” applications like the plant leaves and fuel cells.
NMR Spectroscopy Applied to Proteins:
Much of the recent innovation within NMR spectroscopy has been within the field of protein NMR, which has become a very important technique in structural biology. One common goal of these investigations is to obtain high resolution 3-dimensional structures of the protein, similar to what can be achieved by X-ray crystallography.
In contrast to X-ray crystallography, NMR is primarily limited to relatively small proteins, usually smaller than 25 kDa, though technical advances allow ever larger structures to be solved. NMR spectroscopy is often the only way to obtain high resolution information on partially or wholly intrinsically unstructured proteins.
Proteins are orders of magnitude larger than the small organic molecules discussed earlier in this article, but the same NMR theory applies. Because of the increased number of each element present in the molecule, the basic 1D spectra become crowded with overlapping signals to an extent where analysis is impossible.
Therefore, multidimensional (2, 3 or 4D) experiments have been devised to deal with this problem. To facilitate these experiments, it is desirable to isotopically label the protein with 13C and 15N because the predominant naturally-occurring isotope 12C is not NMR-active, whereas the nuclear quadrupole moment of the predominant naturally-occurring 14N isotope prevents high resolution information to be obtained from this nitrogen isotope.
The most important method used for structure determination of proteins utilizes NOE experiments to measure distances between pairs of atoms within the molecule. Subsequently, the obtained distances are used to generate a 3D structure of the molecule using a computer program.
Type # 12. Laser-Induced Breakdown Spectroscopy:
Laser-induced breakdown spectroscopy (LIBS) is a type of atomic emission spectroscopy which utilizes a highly energetic laser pulse as the excitation source. LIBS can analyse any matter regardless of its physical state, be it solid, liquid or gas. Even slurries, aerosols, gels, and more can be readily investigated. Because all elements emit light when excited to sufficiently high temperatures, LIBS can detect all elements, limited only by the power of the laser as well as the sensitivity and wavelength range of the spectrograph & detector.
Operationally, LIBS is very similar to arc/spark emission spectroscopy. LIBS can often be referred to as its alternative name: laser-induced plasma spectroscopy (LIPS). Unfortunately the term LIPS has alternative meanings that are outside the field of analytical spectroscopy, therefore, the term LIBS is preferred. LIBS is technically very similar to a number of other laser-based analytical techniques, sharing much of the same hardware. These techniques arc the vibrational spectroscopic technique of Raman spectroscopy, and the fluorescence spectroscopic technique of laser-induced fluorescence (LIF).
In fact, devices are now being manufactured which combine these techniques in a single instrument, allowing the atomic, molecular and structural characterisation of a specimen as well as giving a deeper insight into physical properties.
Design:
A typical LIBS system consists of a neodymium doped yttrium aluminium garnet (Nd:YAG) solid state laser and a spectrometer with a wide spectral range and a high sensitivity, fast response rate, time gated detector. This is coupled to a computer which can rapidly process and interpret tin acquired data. As such LIBS is one of the most experimentally simple spectroscopic analytical techniques, making it one of the cheapest to purchase and to operate.
The Nd:YAG laser generates energy in the near infrared region of the electromagnetic spectrum, with a wavelength of 1064 nm. The pulse duration is in the region of 10 ns generating a power density which can exceed 1 GW.cm 2 at the focal point. Other lasers have been used for LIBS mainly Excimer (Excited dimer) type generating energy in the visible and ultraviolet regions. The spectrometer consists of either a monochromator (scanning) or a polychromator (non-scanning) and a photomultiplier or CCD detector respectively.
The most common monochromator is the Czerny-Turner type whilst the most common polychromator is the Echelle type, even so the Czerny-Turner type can be (and s often) used to disperse the radiation onto CCD effectively making it a polychromator. The polychromator spectrometer is the type most commonly used in LIBS as it allows simultaneous acquisition of the entire wavelength range of interest.
The spectrometer collects electromagnetic radiation over the widest wavelength range possible, maximising the number of emission lines detected for each particular element. Spectrometer response is typically from 1100 nm (near infrared) to 170 nm (deep ultraviolet), the approx. mate response range of a CCD detector. All elements have emission lines within this wavelength range.
The energy resolution of the spectrometer can also affect the quality of the LIBS measurement, since high resolution systems can separate spectral emission lines in close juxtaposition, reducing interference and increasing selectivity. This feature is particularly important in specimens which have a complex matrix, containing a large number of different elements.
Accompanying the spectrometer and detector is a delay generator which accurately gates the detector’s response time, allowing temporal resolution of the spectrum. LIBS operates by focusing the laser onto a small area at the surface of the specimen, when the laser is discharged it ablates a very small amount of material, in the range of Nano gram to picogram which instantaneously generates a plasma plume with temperatures of about 10,000-20,000 K.
At these temperatures the ablated material dissociates (breaks down) into excited ionic and atomic species. During this time the plasma emits a continuum of radiation which does not contain any useful information about the species present, but within a very small timeframe the plasma expands at supersonic velocities and cools.
At this point the characteristic atomic emission lines of the elements can be observed. The delay between the emission of continuum radiation and characteristic radiation is in the order of 10 µs, this is why it is necessary to temporally gate the detector.
Advantages:
Because such a small amount of material is consumed during the LIBS process the technique is considered essentially non-destructive or minimally-destructive, and with an average power density of less than one watt radiated onto the specimen there is almost no specimen heating surrounding the ablation site.
Due to the nature of this technique sample preparation is typically minimised to homogenization or is often unnecessary where heterogeneity is to be investigated or where a sped men is known to be sufficiently homogeneous, this reduces the possibility of contamination during chemical preparation steps.
One of the major advantages of the LIBS technique is its ability to depth profile a specimen by repeatedly discharging the laser in the same position, effectively going deeper into the specimen with each shot. This can also be applied to the removal of surface contamination, where the laser is discharged a number of times prior to the analysing shot.
LIBS is also a very rapid technique giving results within seconds, making it particularly useful for high volume analyses or on-line industrial monitoring. LIBS is an entirely optical technique, therefore it requires only optical access to the specimen. This is of major significance as fibre optics can be employed for remote analyses.
And being an optical technique it is non-invasive, non-contact and can even be used as a stand-off analytical technique when coupled to appropriate telescopic apparatus. These attributes have significance for use in areas from hazardous environments to space exploration. Additionally LIBS systems can easily be coupled to an optical microscope for micro-sampling adding a new dimension of analytical flexibility.
The use of specialised optics or a mechanically positioned specimen stage can be used raster the laser over the surface of the specimen allowing spatially resolved chemical analysis and the creation of ‘elemental maps’. This is very significant as chemical imaging is becoming more important in all branches of science and technology.
Portable LIBS systems are more sensitive, faster and can detect a wider range of elements (particularly the light elements) than competing techniques such as portable X-ray fluorescence. And LIBS does not utilise ionizing radiation to excite the sample, which is both penetrating and potentially carcinogenic.
Disadvantages:
LIBS, like all other analytical techniques is not without limitations. LIBS is subject to the matrix effect which can be minimised by good specimen preparation and the use of accurate calibration standards, it is also subject to variation in the laser spark and resultant plasma which often limits reproducibility.
The accuracy of LIBS measurements is typically better than 10% and precision is often better than 5%. The detection limits for LIBS vary from one element to the next depending on the specimen type and the experimental apparatus used. Even so detection limits of 1 to 30 ppm by mass are not uncommon, but can range from >100 ppm to <1 ppm.
Recent interest in LIBS has focused on the miniaturization of the components and the development of compact, low power, portable systems. This direction has been pushed along by interest horn groups such as NASA, ESA as well as the military. The Mars Science Laboratory mission plans to bring combined Raman/LIBS onto Mars.
Recent developments in LIBS have seen the introduction of double-pulsed laser systems. For double-pulse LIBS one distinguishes between orthogonal and perpendicular configuration. In perpendicular configuration the laser is fired twice on the same spot on the specimen with a pulse separation in the order of one to a couple of tens of microseconds.
Depending on pulse separation, the second pulse is more or less absorbed by the plasma plume caused by the previous pulse, resulting in a reheating of the laser plasma leading to signal enhancement. In orthogonal configuration a laser pulse is fired parallel to the sample surface either before or after the perpendicular pulse hits the specimen.
The laser plasma ignited in the surrounding medium above the surface by a first pulse causes (by its shock wave) an area of reduced pressure above the specimen into which the actual plasma from the sample can expand. This has similar positive effects on sensitivity like LIBS performed at reduced pressures.
If the orthogonal laser pulse is delayed with respect to the perpendicular one, the effects are similar as in the perpendicular configuration. Both double pulse LIBS as well as LIBS at reduced pressures are aimed at increasing the sensitivity of LIBS and the reduction of errors caused by the differential volatility of elements (e.g., Hydrogen as an impurity in solids).
It also significantly reduces the matrix effects. Double-pulsed systems are also proving useful in conducting analysis in liquids, as the initial laser pulse forms a cavity bubble in which the second pulse acts on the evaporated material. LIBS is one of several analytical techniques that can be deployed in the field as opposed to pure laboratory techniques, e.g., spark OES. Recent research on LIBS is focusing on compact.
Type # 13. Mossbauer Spectroscopy:
Mossbauer spectroscopy is a spectroscopic technique based on the Mossbauer Effect. In its most common form, Mossbauer Absorption Spectroscopy, a solid sample is exposed to a beam of gamma radiation, and a detector measures the intensity of the beam that is transmitted through the sample, which will change depending on how many gamma-rays are absorbed by the sample.
The atoms m the source emitting the gamma rays are the same as the atoms in the sample absorbing them. Thanks to the Mossbauer effect that a significant fraction of the gamma-rays emitted by the atoms in the source do not lose any energy due to recoil and thus have almost the right energy to be absorbed by the target atoms.
The gamma-ray energy is varied by accelerating the gamma-ray source through a range of velocities with a linear motor. The relative motion between the source and sample results in an energy shift due to the Doppler effect. In the resulting spectra, gamma-ray intensity is plotted as a function of the source velocity.
At velocities corresponding to the resonant energy levels of the sample, some of the gamma-rays are absorbed, resulting in a drop in the measured intensity and a corresponding dip in the spectrum. The number, positions, and intensities of the dips (also called peaks) provide information about the chemical environment of the absorbing nuclei and can be used to characterize the sample.
In order for Mossbauer absorption of gamma- rays to occur, the gamma-ray must be of the appropriate energy for the nuclear transitions of the atoms being probed, which is almost always achieved by having the same atoms of the same isotope in both the source and the target. Also, the gamma-ray energy should be relatively low; otherwise the system will have a low recoil-free fraction (see Mossbauer Effect) resulting in a poor signal-to-noise ratio.
Only a handful of elemental isotopes exist for which these criteria are met, so Mossbauer spectroscopy can only be applied to a relatively small group of atoms including: 57Fe, 129I, 119Sn, and 121Sb. Of these, 57Fe is by far the most common element studied using the technique.
Type # 14. Rotational Spectroscopy:
Rotational spectroscopy or microwave spectroscopy studies the absorption and emission electro-magnetic radiation (typically in the microwave region of the electromagnetic spectrum) by molecules associated with a corresponding change in the rotational quantum number of the molecule. The use of microwaves in spectroscopy essentially became possible due to the development of microwave technology for RADAR during World War II.
Rotational spectroscopy is only really practical in the gas phase where the rotational motion is quantized. In solids or liquids the rotational motion is usually quenched due to collisions. Rotational spectrum from a molecule (to first order) requires that the molecule have a dipole moment, that is a difference between the centre of charge and the centre of mass, or equivalently a separation between two unlike charges.
It is this dipole moment that enables the electric field of the light (microwave) to exert a torque on the molecule causing it to rotate more quickly (in excitation) or slowly (in de-excitation). Diatomic molecules such as Oxygen (O2), Hydrogen (H2), etc. do not have a dipole moment and hence no purely rotational spectrum.
However, electronic excitations can lead to asymmetric charge distributions and thus provide a net dipole moment to the molecule. Under such circumstances, these molecules will exhibit a rotational spectrum. Amongst the diatomic molecules, Carbon monoxide (CO) has one of the simplest rotational spectra.
As for tri-atomic molecules, Hydrogen cyanide (HC≡N) has a simple rotational spectrum for a linear molecule and Hydrogen isocyanides (HN=C:) for a non-linear molecule. As the number of atoms increases the spectrum becomes more complex as lines due to different transitions start overlapping.
Understanding the Rotational Spectrum:
In quantum mechanics the free rotation of a molecule is quantized, that is the rotational energy and the angular momentum can only take certain fixed values; what these values are is simply related to the moment of inertia, I, of the molecule. In general for any molecule, there are three moments of inertia: IA, IB and IC about three mutually orthogonal axes A, B, and C respectively with the origin at the center of mass of the system.
A linear molecule is a special case in this regard. These molecules are cylindrically symmetric and one of the moment of inertia (IA, which is the moment of inertia f or a rotation taking place along the axis of the molecule) is negligible (i.e. IA << IB = IC)
Classification of Molecules Based on Rotational Behaviour:
The general convention is to define the axes such that the axis A has the smallest moment of inertia (and hence the highest rotational frequency) and other axes such that IA < IB < IC. Sometimes the axis A may lie associated with the symmetric axis of the molecule, if any. If such is the case, then IA need not be the smallest moment of inertia. To avoid confusion, we will stick with the former convention tor the rest of the article. The particular pattern of energy levels (and hence of transitions in the rotational spectrum) for a molecule is determined by its symmetry. A convenient way to look at the molecules is to divide them into four different classes (based on the symmetry of their structure).
These are:
1. Linear molecules (or linear rotors):
For a linear molecule IA << IB = IC. For most of the purposes, IA is taken to be zero. For a linear molecule, the separation of lines in the rotational spectrum can be related directly to the moment of inertia of the molecule, and for a molecule of known atomic masses, can be used to determine the bond lengths (structure) directly.
For diatomic molecules this process is trivial, and can be made from a single measurement of the rotational spectrum. For linear molecules with more atoms, rather more work is required, and it is necessary to measure molecules in which more than one isotope of each atom have been substituted (effectively this gives rise to a set of simultaneous equations which can be solved for the bond lengths).
Examples or linear molecules:
Oxygen (O=O), Carbon monoxide (O≡C*), Hydroxy radical (OH), Carbon dioxide (O=C=O), Hydrogen cyanide (HC≡N), Carbonyl sulphide (O=C=S), Chloroethyne (HC≡CCI), Acetylene (HC≡CH).
2. Symmetric tops (or symmetic rotors):
A symmetric top is a molecule in which two moments of inertia are the same. As a matter of historical convenience, spectroscopists divide molecules into two classes of symmetric tops, Oblate symmetric tops (saucer or disc shaped) with IA = IB < IC and Prolate symmetric tops (rugby football, or cigar shaped) with IA < IB = IC. The spectra look rather different, and are instantly recognizable. As for linear molecules, the structure of symmetric tops (bond lengths and bond angles) can be deduced from their spectra.
Examples of symmetric tops:
Oblate: Benzene (C6H6), Cyclobutadiene (C4H4), and Ammonia (NH3) Prolate: (Chloroform (CHCI3), Propyne (CH3C≡CH).
3. Spherical tops (or spherical rotors):
A spherical top molecule, can be considered as a special case of symmetric tops with equal moment of inertia about all three axes (IA = IB = IC).
Examples of spherical tops:
Phosphorus tetramer (P4), (Carbon tetrachloride (CCI4), Nitrogen tetra hydride (NH4), Ammonium ion (NH+4), Sulphur hexafluoricle (SF6).
4. Asymmetric tops:
As you would have guessed a molecule is termed an asymmetric top if all three moments of inertia are different. Most of the larger molecules are asymmetric tops, even when they have a high degree of symmetry. Generally tor such molecule a simple interpretation of the spectrum is not normally possible.
Sometimes asymmetric tops have spectra that are similar to those of a linear molecule or a symmetric top, in which case the molecular structure must also be similar to that of a linear molecule or a symmetric top. For the most general case, however, all that can be done is to fit the spectra to three different moments of inertia.
It the molecular formula is known, then educated guesses can be made of the possible structure, and from this guessed structure, the moments of inertia can be calculated. If the calculated moments of inertia agree well with the measured moments of inertia, then the structure can be said to have been determined. For this approach to determining molecular structure, isotopic substitution is invaluable.
Examples of asymmetric tops:
Anthracene (C14H10), Water (H2O), Nitrogen dioxide (NO2). A molecule is always in vibration. As the molecule vibrates, its moment of inertia changes. Further there is a fictitious force, Coriolis coupling, between the vibrational motions of the nuclei in the rotating (non-inertial) frame.
However, as long as the vibrational quantum number does not change (i.e., the molecule is in only one state of vibration), the effect of vibration on rotation is not important, because the time for vibration is much greater than the time required for rotation. The Coriolis coupling is often negligible, too, if one is interested in low vibrational and rotational quantum numbers only.
Hyperfine Interactions:
In addition to the main structure that is observed in microwave spectra due to the rotational motion of the molecules, a whole host of further interactions are responsible for small details in the spectra, and the study of these details provides a very deep understanding of molecular quantum mechanics. The main interactions responsible for small changes in the spectra (additional splitting’s and shifts of lines; are due to magnetic and electrostatic interactions in the molecule.
The particular strength of such interactions differs in different molecules, but in general, the order of these effects (in decreasing significance) is:
i. Electron spin — electron spin interaction (this occurs in molecules with two or more unpaired electrons, and is a magnetic-dipole/magnetic-dipole interaction)
ii. Electron spin — molecular rotation (the rotation of a molecule corresponds to a magnetic dipole, which interacts with the magnetic dipole moment of the electron)
iii. Electron spin — nuclear spin interaction (the interaction between the magnetic dipole moment of the electron and the magnetic dipole moment of the nuclei, if present)
iv. Electric field gradient — nuclear electric quadrupole interaction (the interaction between the electric field gradient of the electron cloud of the molecule and the electric quadrupole moments of nuclei, if present).
v. Nuclear spin — nuclear spin interaction (nuclear magnetic moments interacting with one another).
These interactions give rise to the characteristic energy levels that are probed in “magnetic resonance” spectroscopy such as NMR and HSR, where they represent the “zero field splitting’s” which are always present. Fourier transform infrared (FTIR) spectroscopy can be used to experimentally study rotational spectrum.
Applications:
Microwave spectroscopy is commonly used in physical chemistry to determine the structure of small molecules (such as ozone, methanol, or water) with high precision. Other common techniques for determining molecular structure, such as X-ray crystallography do not work very well for some of these molecules (especially the gases) and are not as precise. However, microwave spectroscopy is not useful tor determining the structures of large molecules such as proteins.