Principles of Spectroscopy:
Spectroscopy is the study of the interaction of electromagnetic radiation with matter. When matter is energized (excited) by the application of thermal, electrical, nuclear or radiant energy, electromagnetic radiation is often emitted as the matter relaxes back to its original (ground) state.
The spectrum of radiation emitted by a substance that has absorbed energy is called an emission spectrum and the science is appropriately called emission spectroscopy.
Another approach often used to study the interaction of electromagnetic radiation with matter is one whereby a continuous range of radiation (e.g., white light) is allowed to fall on a substance; then the frequencies absorbed by the substance are examined.
The resulting spectrum from the substance contains the original range of radiation with dark spaces that correspond to missing, or absorbed frequencies. This type of spectrum is called an absorption spectrum. In spectroscopy the emitted or absorbed radiation is usually analyzed, i.e., separated into the various frequency components and the intensity is measured by means of an instrument called spectrometer.
The resultant spectrum is mainly a graph of intensity of emitted or absorbed radiation versus wavelength or frequency. There are in general three types of spectra: continuous, line, and band. The sun and heated solids produce continuous spectra in which the emitted radiation contains all frequencies within a region of the electromagnetic spectrum. A rainbow and light from a light bulb are examples of continuous spectra.
Line spectra are produced by excited atoms in the gas phase and contain only certain frequencies, all other frequencies being absent. Each chemical element of the periodic chart has a unique and, therefore, characteristic line spectrum. Band spectra are produced by excited molecules emitting radiation in groups of closely spaced lines that merge to form bands.
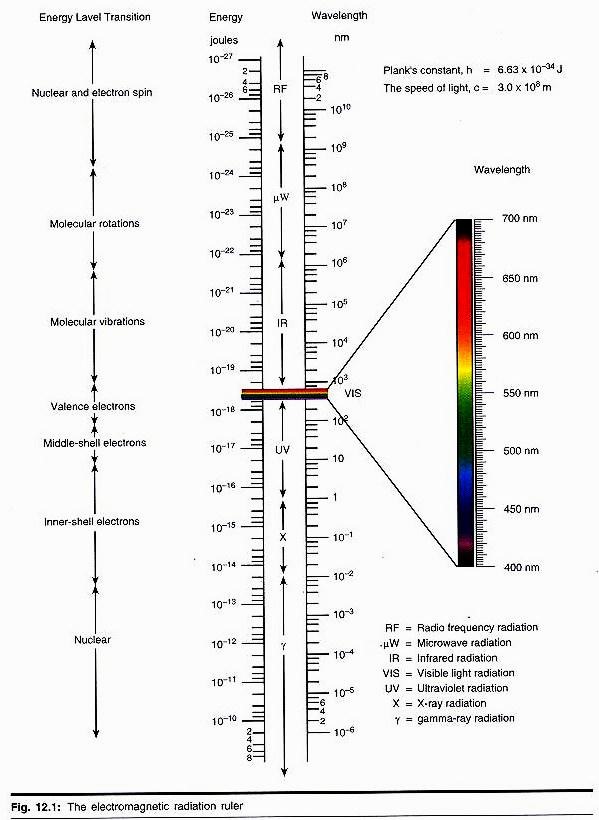
These categories of emission and absorption spectra contain tremendous amounts of useful information about the structure and composition of matter. Spectroscopy is a powerful and sensitive form of chemical analysis, as well as a method of probing electronic and nuclear structure and chemical bonding. The key to interpreting this spectral information is the knowledge that certain atomic and molecular processes involve only certain energy ranges. Fig. 12.1 shows the regions of the electromagnetic spectrum and the associated energy transitions that occur in atomic and molecular processes.
Much of the scientific knowledge of the structure of the universe, from stars to atoms, is derived from interpretations of the interaction of radiation with matter. One example of the power of these techniques is the determination of the composition, the velocities, and the evolutionary dynamics of stars.
The source of the incredible amount of energy produced by the sun is nuclear fusion reactions going on within the hot interior (temperature 40 × 106 K). Two fusion cycles, the carbon cycle and the proton cycle, convert hydrogen nuclei into helium nuclei via heavier nuclei, such as carbon 12 and nitrogen 14. The enormous radiation of energy from the hot core seethes outwards by convection.
This radiation consists of the entire electromagnetic spectrum as a continuous spectrum. Towards the surface of the sun (the photosphere), the different elements all absorb at their characteristic frequencies. The radiation that shoots into space toward the earth is a continuous emission spectrum with about 22,000 dark absorption lines present in it (Fraunhofer lines), of which about 70% have been identified. These absorption lines, i.e., missing frequencies, prove that more than 60 terrestrial elements are certainly present in the sun.
Classification of Spectroscopic Methods:
Different spectroscopic techniques have been classified mainly on two parameters, first what type of radiation is to be measured or by what measurement procedure is employed.
1. Nature of Radiation Measured:
This category of spectroscopy depends on the physical quantity measured. Normally, the quantity that is measured is an amount or intensity of something.
i. Electromagnetic spectroscopy involves interactions with electromagnetic radiation, or light. Ultraviolet-visible spectroscopy is an example.
ii. Electronic spectroscopy involves interactions with electron beams. Auger spectroscopy involves inducing the Auger effect with an electron beam.
iii. Mechanical spectroscopy involves interactions with macroscopic vibrations, such as phonons. An example is acoustic spectroscopy, involving sound waves.
iv. Mass spectroscopy involves the interaction of charged species with a magnetic field, giving rise to a mass spectrum. The term “mass spectroscopy” is deprecated in favour of mass spectrometry, tor the technique is primarily a form of measurement, though it doe- produce a spectrum for observation.
2. Measurement Process:
Most spectroscopic methods are differentiated as either atomic or molecular based on whether or not they apply to atoms or molecules.
Along with that distinction, they can be classified on the nature of their interaction:
i. Absorption spectroscopy uses the range of the electromagnetic spectra in which a sub stance absorbs. This includes atomic absorption spectroscopy and various molecular techniques, such as infrared spectroscopy in that region and nuclear magnetic resonance (NMR) spectroscopy in the radio region.
ii. Emission spectroscopy uses the range of electromagnetic spectra in which a substance radiates (emits). The substance first must absorb energy. This energy can be from a variety of sources, which determines the name of the subsequent emission, like luminescence. Molecular luminescence techniques include spectrofluorimetry.
iii. Scattering spectroscopy measures the amount of light that a substance scatters at certain wavelengths, incident angles, and polarisation angles. The scattering process is much faster than the absorption/emission process. One of the most useful applications of light scattering spectroscopy is Raman spectroscopy.