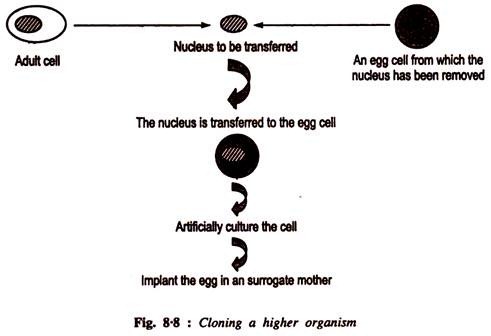
This article throws light upon the three methods used for separation of particles in centrifugation.
The three methods are: (1) Differential Centrifugation (2) Centrifugal Elutriation and (3) Density Gradient Centrifugation. The Density Gradient Centrifugation method consists of two techniques. The two techniques are: (1) Rate Zonal Technique and (2) Isopycnic Centrifugation Technique.
Contents
Method # 1. Differential Centrifugation:
This depends upon the sedimentation rate of particles of different size and density. Centrifugations will initially sediment the largest particles.
For particles with same mass but with different densities, the one with highest density will sediment first. Particles having similar banding densities can usually be efficiently separated one from another by differential centrifugation or rate zonal method, provided that there are at least 10-fold differences in their masses.
In differential centrifugation the material to be separated is divided centrifugally into number of fractions by increasing the applied centrifugal field. The centrifugal field at each step is chosen so that particular type of material sediments. Any type of particle originally present in homogenate may be found in pellet or the supernatant or both fractions, depending upon the time and speed of centrifugation and size and density of particles. At the end of each stage the pellet and supernatant are separated and pellet washed several times by re-suspension and re-centrifugation in homogenation medium.
Initially all particles of homogenate are homogenously distributed throughout the centrifuge tube. During centrifugation particles move down the centrifuge tubes at their respective sedimentation rates and start to form a pellet on the bottom of centrifuge tube. Ideally centrifugation is continued enough to pellet all the largest class of particles, the resulting supernatant then being centrifuged at a higher speed to separate medium-sized particles and so on.
However, since particles of varying sizes and densities were distributed homogenously at the commencement of centrifugation, it is evident that the pellet will not be homogenous but will contain a mixture of all the sedimented components, being enriched with fastest sedimenting particles. In the time required for complete sedimentation of heavier particles, some of the lighter and medium sized particles, originally suspended near the bottom of the tube, will also sediment and thus contaminate the fraction.
Pure preparation of the pellet of the heaviest particle cannot be, therefore, obtained in single centrifugation step. It is only the most slowly sedimenting component of mixture remaining in the supernatant after all the larger particles have been sedimented that can be purified by single centrifugation step.
The separation achieved by differential centrifugation can be improved by repeated re-suspension and re-centrifugation under similar condition. Further centrifugation of the supernatant with gradually increasing centrifugal fields results in sedimentation of intermediate and finally the smallest and least dense particles. In spite of its inherent limitations, differential centrifugation is probably the most commonly employed method for isolation of cell organelles from, homogenized tissue.
Method # 2. Centrifugal Elutriation:
In this technique the’ separation and purification of a large variety of cells from different tissues and species can be achieved by a gentle “washing action” using an elutriator rotor. The technique is based upon the differences in the set-up in separation chamber of rotor, between the opposing centripetal liquid flow and applied centrifugal field being used to separate particles mainly on the basis of differences in their size.
The technique does not employ the density gradient and have advantage that any medium totally compatible with the particles can be used. By this separation can be achieved very quickly, giving high cell concentrations and a very good recovery yield.
Method # 3. Density Gradient Centrifugation:
There are two methods of density gradient centrifugation, the rate zonal technique and the isopycnic (iso-density or equal density) technique, and both can be used when quantitative separation of all the components of mixture of particles is required. They are also used for the determination of buoyant densities and for the estimation of sedimentation coefficient.
(a) Rate Zonal Technique:
Particle separation by the rate zonal technique is based upon differences in the size, shape and density of particles, the density and viscosity of the medium and the applied centrifugal field. Subcellular organelles, which have different densities but are similar in size, do not separate efficiently using this method, but separation of proteins of similar densities and differing only 3 folds in relative molecular mass can be achieved easily.
The technique involves carefully layering a sample solution on top of preformed liquid density gradient, the highest density of which does not exceed that of densest particle to be separated. The function of gradient is primarily to stabilize the liquid column in the tube against the movements resulting from conventional currents and secondarily to produce a gradient that helps to improve the resolution of gradient.
The sample is then centrifuged until the desired degree of separation is achieved. Since the technique is time dependent, centrifugation must be terminated before any of the separated zone pellets at he bottom of tube. The technique is employed for the separation of enzymes, RNA-DNA hybrids, ribosomal subunit, subcellular organelle, etc.
(b) Isopycnic Centrifugation Technique:
Isopycnic centrifugation depends solely upon the buyout density and not on its shape, size and time, the size of the particle affecting only the rate at which it reaches its isopycnic position in the gradient. The technique is used to separate particles of similar size but of different density. Hence soluble proteins which have very similar densities cannot be usually separated by this method, where as sub cellular organelles can be effectively separated.
The methods are a combination of sedimentation and flotation and involve layering the sample on top of a density gradient that spans the whole range of the particle densities that are to be separated. The maximum density of the gradient, therefore, must always exceed the density of the densest particle. During centrifugation, sedimentation of the particle occurs until the buoyant density of the particle and density of the gradient are equal.
At this point of isodensity no further sedimentation occurs, irrespective of how long centrifugation continues, because the particles are floating on the cushion of material that has density greater than their own. Isopycnic centrifugation, in contrast to the rate zonal technique, is an equilibrium method, the particle banding to form zones each at their own characteristic buoyant density.
In case when not all components in a mixture of particle are required, a gradient range can be selected in which unwanted materials will be sediment at the bottom of the tube and whole of the particles of interest will float at their respective isopynic positions. Such a technique involves a combination of both the rate zonal and isopynic approaches.
Features of Gradient Material:
There is no ideal all purpose gradient material; the choice of solute depends upon the nature of the particles to be fractionated.
The gradient material should:
i. Permit the desire type of separation
ii. Be stable in solution
iii. Be inert towards biological material and not react with the centrifuge , rotor, tubes or caps:
iv. Not absorb light at wavelengths appropriate for spectrophotometric monitoring (visible or ultraviolet range), or otherwise interfere with assaying procedures;
v. Be sterilisable, non-toxic or flammable;
vi. Have negligible osmotic pressure and cause minimum changes in ionic strength, pH and viscosity;
vii. Be inexpensive and readily available in pure form and capable of forming a solution covering the density range needed for a particular application without overstressing the rotor;
viii. Allow easy separation of the sample material from the gradient medium with loss of the sample or its activity.
Generally used gradient materials are salts of alkali metals (e.g., caesium and rubidium chloride), small neutral hydrophilic organic molecules (e.g., sucrose), hydrophilic macromolecules (e.g., proteins and polysaccharides), and a number of miscellaneous compounds such as colloidal silica (e.g., percoll and ludox) and non-ionic iodinated aromatic compounds (e.g., metrizamide, nycodenz and renograffin).
Sucrose solution while suffering from disadvantages of being very viscous at densities greater than 1.1 to 1.2 g cm-3 and exerting very high osmotic effects even at very low concentrations have been found to be most convenient gradient material for rate zonal separation. Glycerol is also used as gradient material especially for the separation of enzymes, or alternative media such as ficoll, metrizamide or percoll gradients may be utilized.
Non-ionic media, such as sucrose, glycerol, metrizamide, ficoll and percoll are generally considered to be gender than ionic salts like caesium chloride and potassium bromide and require a lower centrifugal fields to achieve adequate separation of particles. In case of isopycnic separation, no one medium has proved satisfactory for the isolation of all type of biological particles.
Ficoll has successfully used for the separation of whole cell and subcellular organelles by rate zonal and isopycnic centrifugations. Caesium and rubidium salts are used exclusively for isopycnic separations and have been used most frequently for separation of high density solutes like nucleic acids.
However, at high concentrations their high ionic strength and osmolarity tends to disrupt intra- and inter-molecular bonds. Generally, ionic media have been used for the separation of nucleic acids, proteins and viruses and non-ionic iodinated aromatic compounds, because of their increased versatility, have been used for a much wider variety of applications.