This article throws light upon the top seven types of antibiotics. The seven types of antibiotics are: (1) Penicillins (2) Cephalosporin’s (3) Aminoglycosides (4) Tetracyclines (5) Macrolides (6) Aromatic Antibiotics and (7) Nucleoside Antibiotics.
Contents
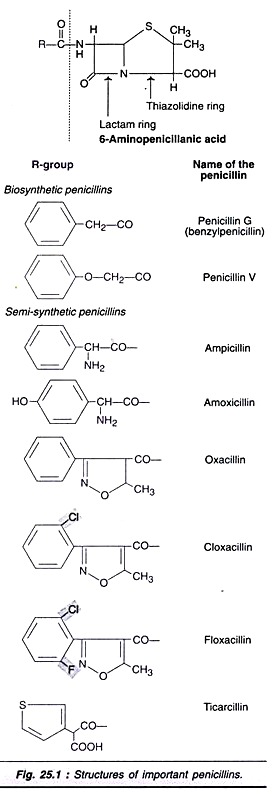
Type # 1. Penicillins:
Penicillins are a group of β-lactam containing bactericidal antibiotics. Being the first among the antibiotics to be discovered, penicillins are historically important. The structures of important synthetic and semi-synthetic penicillins are depicted in Fig. 25.1. The basic structure of all the penicillins consists of a lactam ring and a thizolidine ring fused together to form 6-aminopenicillanic acid.
Action of Penicillins:
Natural penicillins (penicillins V and G) are effective against several Cram-positive bacteria. They inhibit the bacterial cell wall (i.e. peptoglycan) synthesis and cause cell death. Some persons (approximately 0.5-2% of population) are allergic to penicillin.
Natural penicillins are ineffective against microorganisms that produce β-lactamase, since this enzyme can hydrolyse penicillins e.g. Staphylococcus aureus. Several semi-synthetic penicillins that are resistant to β-lactamase have been developed and successfully used against a large number of Gram-negative bacteria.
Cloxacillin, ampicillin, floxacillin and azlocillin are some examples of semi-synthetic penicillins. These are quite comparable in action to cephalosporin’s. From the huge quantities of penicillins produced by fermentation, about 40% are used for human healthcare, 15% for animal healthcare and 45% for the preparation of semi-synthetic penicillins.
Organisms for Penicillin Production:
In the early days, Penlcillium notatum was used for the large-scale production of penicillins. Currently, Penicillium chrysogenum and its improved mutant strains are preferred. Previously, the penicillin production used to be less than 2 units/ml, and with the new strains, the production runs into several thousands of units/ml. One of the high yielding strains wis Q176 is preferred by several penicillin manufacturers.
Genetic engineering for improved penicillin production:
Some of the genes involved in penicillin biosynthesis by P. chrysogenum have been identified. Genetic manipulations were carried out so as to substantially increase the penicillin production. For instance, extra genes coding for the enzymes cyclase and acyltransferase have been inserted into C. chrysogenum.
Biosynthesis of Penicillin:
L-α-Aminoadipic acid combines with L-cysteine, and then with L-valine to form a tripeptide namely α-L-aminoadipylcysteinylvaline. This compound undergoes cyclization to form isopenicillin which reacts with phenyl acetyl CoA (catalysed by the enzyme acyltransferase) to produce penicillin G (benzyl penicillin). In this reaction, aminoadipic acid gets exchanged with phenylacetic acid (Fig. 25.2).
Regulation of biosynthesis:
Some of the biochemical reactions for the synthesis of penicillin and lysine are common. Thus, L-α-aminoadipic acid is a common intermediate for the synthesis of penicillin and lysine. The availability of aminoadipic acid plays a significant role in regulating the synthesis of penicillin.
Penicillin biosynthesis is inhibited by glucose through catabolite repression. For this reason, penicillin was produced by a slowly degraded sugar like lactose. The concentrations of phosphate and ammonia also influence penicillin synthesis.
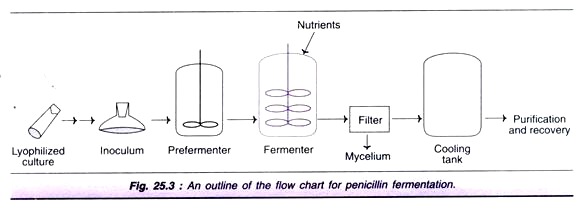
Production Process of Penicillin:
An outline of the flow chart for the industrial production of penicillin is depicted in Fig. 25.3. The lyophilized culture of spores is cultivated for inoculum development which is transferred to pre-fermenter, and then to fermenter.
Penicillin production is an aerobic process and therefore, a continuous supply of O2 to the growing culture is very essential. The required aeration rate is 0.5-1.0 vvm. The pH is maintained around 6.5, and the optimal temperature is in the range of 25-27°C. Penicillin production is usually carried out by submerged processes. The medium used for fermentation consists of corn steep liquor (4-5% dry weight) and carbon source (usually lactose). An addition of yeast extract, soy meal or whey is done for a good supply of nitrogen.
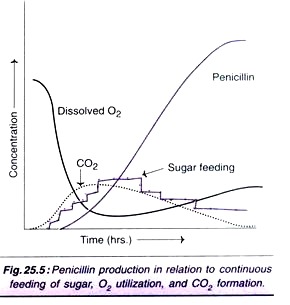
Sometimes, ammonium sulfate is added for the supply of nitrogen. Phenylacetic acid (or phenoxyacetic acid) which serves as a precursor for penicillin biosynthesis is continuously fed. Further, continuous feeding of sugar is advantageous for a good yield of penicillin. The penicillin production profiles are depicted in Figs. 25.4 and Fig. 25.5.
It is estimated that approximately 10% of the metabolised carbon contributes to penicillin production, while 65% is utilised towards energy supply and 25% for growth of the organisms. The efficiency of penicillin production can be optimized by adequate supply of carbon source. Thus, by adding glucose and acetic acid, the yield can be increased by about 25%.
For efficient synthesis of penicillin, the growth of the organism from spores must be in a loose form and not as pellets. The growth phase is around 40 hours with a doubling time of 6-8 hours. After the growth phase is stabilized, the penicillin production exponentially increases with appropriate culture conditions. The penicillin production phase can be extended to 150-180 hours.
Recovery of Penicillin:
As the fermentation is complete, the broth containing about 1% penicillin is processed for extraction. The mycelium is removed by filtration. Penicillin is recovered by solvent (n-butyl acetate or methyl ketone) extraction at low temperature (<10°C) and acidic pH (<3.0). By this way, the chemical and enzymatic (bacterial penicillinase) degradations of penicillin can be minimized.
The penicillin containing solvent is treated with activated carbon to remove impurities and pigments. Penicillin can be recovered by adding potassium or sodium acetate. The potassium or sodium salts of penicillin can be further processed (in dry solvents such as n-butanol or isopropanol) to remove impurities. The yield of penicillin is around 90%.
As the water is totally removed, penicillin salts can be crystallized and dried under required pressure. This can be then processed to finally produce the pharmaceutical dosage forms. Penicillins G and H are the fermented products obtained from the fungus Penicillium chrysogenum.
Production of 6-Amino Penicillanic Acid:
The penicillins G and H are mostly used as the starting materials for the production of several synthetic penicillins containing the basic nucleus namely 6-amino penicillanic acid (6-APA). About 10 years ago, only chemical methods were available for hydrolysis of penicillins to produce 6-APA. Now a days, enzymatic methods are preferred.
Immobilized penicillin amidases enzymes have been developed for specific hydrolysis of penicillin G and penicillin V. Penicillin salt of either G or V can be used for hydrolysis by immobilized enzyme system. The pH during hydrolysis is kept around 7-8, and the product 6-APA can be recovered by bringing down the pH to 4.
At pH 4, 6-amino penicillanic acid gets precipitated almost completely in the presence of a water immiscible solvent. In general, the enzymatic hydrolysis is more efficient for penicillin V than for penicillin G. However, penicillin G is a more versatile compound, as it is required for ring expansions.
Type # 2. Cephalosporin’s:
The pharmaceutical uses of penicillins are associated with allergic reactions in some individuals. To overcome these allergic problems, cephalosporin’s were developed. They have improved stability against β-lactamases, and are more active against Gram-negative bacteria. Cephalosporin’s are broad spectrum antibiotics with low toxicity. The structures of different cephalosporin’s are shown in Fig. 25.6. Basically cephalosporin’s have a β-lactam ring fused with a dihydrothiazine ring.
Organisms for Cephalosporin Production:
Cephalosporin C was first discovered in the cultures of fungus Cephalosporium acremonium (later renamed as Acremonium chreysogenum) and this organism continuous to be used even today. The other organisms employed for cephalosporin production are Emericeliopsis sp, Paecilomyces sp and Streptomyces sp.
Several mutants of C. acremonium have been developed for improved production of cephalosporin. Mutants with defective sulfur metabolism or those with resistance to sulfur analogs have high yielding capacity. Certain regulatory genes of cephalosporin biosynthesis (e.g., isopenicillin N synthetase) have been cloned and genetic manipulations carried out for increased production of cephalosporin’s.
Biosynthesis of Cephalosporin:
The early stages of the biosynthetic pathway for cephalosporin are the same as that for penicillin synthesis (See Fig. 25.2). As the tripeptide (aminoadipylcysteinylvaline) is formed, it undergoes cyclization to produce isopenicillin N. By the action of epimerase, penicillin N is formed from isopenicillin N. Then, penicillin N gets converted to cephalosporin C by a three stage reaction catalysed by three distinct enzymes namely expandase, hydroxylase and acetyl transferase (Fig. 25.7).
Regulation of biosynthesis:
A low concentration of lysine promotes cephalosporin synthesis. The inhibitory effect of lysine at a higher concentration can be overcome by adding L-aminoadipic acid. The carbon sources that get rapidly degraded (e.g. glucose, glycerol) reduce cephalosporin production. Methionine promotes cephalosporin synthesis in C. acremonium, but has no influence on Streptomyces’s.
Production Process of Cephalosporin:
The fermentation process concerned with the production of cephalosporin is similar to that of penicillin. The culture media consists of corn steep liquor and soy flour-based media in a continuous feeding system. The other ingredients of the medium include sucrose, glucose and ammonium salts. Methionine is added as a source of sulfur.
The fermentation is carried out at temperature 25-28°C and pH 6-7. The growth of microorganisms substantially increases with good O2 supply, although during production phase, O2 consumption declines. Cephalosporin C from the culture broth can be recovered by ion-exchange resins, and by using column chromatography. Cephalosporin C can be precipitated as zinc, sodium or potassium salt, and isolated.
Chemical synthesis of cephalosporin:
In recent years, by using penicillin V as the starting material, chemical synthesis of cephalosporin has become possible. This is being done due to low cost of production of penicillin.
Production of 7-Aminocephalosporanic Acid:
7-Aminocephalosporonic acid (7-ACA) is the nucleus structure present in all the cephalosporin’s. Cephalosporin C, produced by fermentation, can be subjected to chemical hydrolysis to form 7-ACA. This is tedious, and is associated with several drawbacks.
Recently, enzymatic hydrolysis of cephalosporin C to 7-ACA has been developed. This is mainly carried out by two enzymes-D-amino acid oxidase (isolated from Trigonopsis variabilis) and glutaryl amidase (source-Pseudomonas sp). Bio- technologists have been successful in immobilizing these two enzymes for efficient and large scale manufacture of 7-ACA.
New β-Lactam Technology for Production of 7-ACA:
Scientists have been successful in producing 7-aminocephalosporanic acid by P. chrysogenum fermentation. This is possible through genetic manipulations. As already described in cephalosporin biosynthesis (Fig. 25.7), penicillin N is the substrate for the enzyme expandase. Adipyl- 6-aminopenicillanic acid (produced by P. chrysogenum on adding adipic acid) which resembles in structure with penicillin N, can also serve as a substrate for expandase.
By inserting expandase and hydroxylase gene (cefEF), and acetyl transferase gene (cefG) from S. clavuligerus into P. chrysogenum, the production of adipyl-7-ACA has become possible (Fig. 25.8). Further, the genes responsible for the enzymes D-amino acid oxidase (from Pseudomonas diminuta) have also been inserted into P. chrysogenum. Both these enzymes act on adipyl-7-ACA to produce 7-amino- cephalosporanic acid.
Type # 3. Aminoglycosides:
Aminoglycosides are oligosaccharide (carbohydrate) antibiotics. They contain an aminocyclo-hexanol moiety which is bound to other amino sugars by glycosidic linkages. More than 100 aminoglycosides are known e.g. streptomycin, neomycin, kanamycin, gentamicin, hygromycin, sisomicin.
Aminoglycosides are very potent antibiotics and act against Gram-positive and Gram-negative bacteria, besides mycobacteria. At the molecular level, aminoglycosides bind to 30S ribosome and block protein biosynthesis. Prolonged use of aminoglycosides causes damage to kidneys, and hearing impairment.
For the treatment of severe and chronic infections, aminoglycosides are the antibiotics of choice. Streptomycin was the first aminoglycoside that was successfully used to treat tuberculosis (i.e. against Mycobacterium tuberculosis). Usually, aminoglycosides are regarded as reserve antibiotics, since resistance may develop easily.
Organisms for Aminoglycoside Production:
Aminoglycoside antibiotics are produced by Actinomyces sp. Some examples are given in Table 25.2. Recombinant DNA techniques have been used to produce hybrid aminoglycosides, and for increasing the fermentation yield.
Biosynthesis of Aminoglycosides:
All the ring structures in the molecules of aminoglycosides are ultimately derived from glucose. Most of the biosynthetic pathways concerned with the formation of at least some aminoglycosides have been elucidated.
Biosynthesis of Streptomycin:
The outline of the pathway for the synthesis of streptomycin is depicted in Fig. 25.9. More than 30 enzymatic steps have been identified. Glucose 6-phosphate obtained from glucose takes three independent routes to respectively produce streptidine 6-phosphate, L-dehydrostreptose and N- methyl glucosamine.
The former two compounds condense to form an intermediate which later combines with methyl glucosamine to produce di-hydro-streptomycin-6-phosphate. This compound, in the next of couple of reactions, gets converted to streptomycin.
Regulation of biosynthesis:
Very little is known about the regulation of streptomycin synthesis. A compound named as factor A (chemically isocapryloyl-hydroxymethyl-γ-butyrate) has been isolated from streptomycin-producing strains of S. griseus. Factor A promotes streptomycin production. In fact, factor A- mutants that cannot synthesize streptomycin have been isolated. They can synthesize streptomycin on adding factor A. The nutrient sources-carbohydrates (glucose), ammonia and phosphate also regulate (by feedback mechanism) streptomycin production.
Production Process of Streptomycin:
The medium used for streptomycin usually consists of soy meal or soy flour or corn syrup that can supply glucose at a slow rate (amylase activity is poor in Streptomyces sp). The initial supply of nitrogen (NH3) and phosphate is also obtained from soy meal. This is required since glucose, ammonia and phosphate in high quantities inhibit streptomycin synthesis.
The fermentation conditions for optimal production of streptomycin are — temperature 27-30°C, pH 6.5-7.5, aeration rate 0.5-1.0 vvm. The duration of fermentation process depends on the strain used, and is between 6 to 8 days.
Recovery of Streptomycin:
Streptomycin or other aminoglycosides are basic in nature. They can be recovered by weak cationic exchange resins in an ion- exchange column. Treatment with activated carbon is often necessary to remove impurities. Streptomycin can be precipitated in the form of sulfate salt.
Type # 4. Tetracyclines:
Tetracyclines are broad spectrum antibiotics with widespread medical use. They are effective against Gram-positive and Gram-negative bacteria, besides other organisms (mycoplasmas, chlamydias rickettsias). Tetracyclines are used to combat stomach ulcers (against Helicobacter pylori). They are the most commonly used antibiotics, next to cephalosporin’s and penicillins. Tetracyclines inhibit protein biosynthesis by blocking the binding of aminoacyl tRNA to ribosomes (A site).
The basic structure of tetracyclines is composed of a naphthalene ring (a four ring structure). The substituent groups of the common tetracyclines are given in Fig. 25.10. Among these, chlortetracycline and oxy-tetracycline are most commonly used in the treatment of human and veterinary diseases, besides in the preservation of fish, meat and poultry (in some countries).
Organisms for Tetracycline Production:
The first tetracycline antibiotic that was isolated was chlortetracycline from the cultures of Streptomyces aureofaciens (in 1945). There are at least 20 streptomycetes identified now that usually produces a mixture of tetracyclines. In the Fig. 25.10, a selected list of these organisms for producing tetracyclines is also given.
High-yielding strains of S. aureofaciens and S. rimosus have been developed by using ultraviolet radiation and/or other mutagens (nitrosoguanidine). Such strains are very efficient for the production of chlortetracycline. Further, genetically engineered strains of S. rimosus have been developed for increased synthesis of oxytetracycline.
Biosynthesis of Tetracyclines:
The pathway for the biosynthesis of tetracyclines is very complex. An outline of the synthesis of chlortetracycline by S. aureofaciens is given in Fig. 25.11. There are at least 72 intermediates formed during the course of chlortetracycline biosynthesis, some of them have not been fully characterized.
Polyketide antibiotic synthesis:
The term polyketide refers to a group of antibiotics that are synthesized by successive condensation of small carboxylic acids such as acetate, butyrate, propionate and malonate. The synthesis of polyketide antibiotics is comparable to that of long chain fatty acids. That is the carbon chain grows by cyclic condensation process. The synthesis of tetracyclines is a good example of polyketide antibiotic synthesis.
As glucose gets oxidised, it forms acetyl CoA and then malonyl CoA. On transamination, the later gives malonomoyl CoA. The enzyme anthracene synthase complex binds to malonomoyl CoA and brings out the condensation of 8 molecules of malonyl CoA to form a polyketide intermediates (four ring structures). These intermediates undergo a series of reactions to finally produce chlortetracycline.
Regulation of biosynthesis:
Carbohydrate metabolism (particularly glycolysis) controls chlortetracycline synthesis. For more efficient synthesis of the antibiotic, glycolysis has to be substantially low. The addition of phosphate reduces chlortetracycline production.
Production Process of Chlortetracycline:
The fermentation medium consists of corn steep liquor, soy flour or peanut meal for the supply of nitrogen and carbon sources. Continuous feeding of carbohydrate is desirable for good growth of the organism and production of the antibiotic. This can be done either by addition of crude carbon sources or by supplying glucose or starch. For more efficient production of chlortetracycline, the supply of ammonium and phosphate has to be maintained at a low concentration.
An outline of the production process for chlortetracycline is depicted in Fig. 25.12. The ideal fermentation conditions are — temperature 27-30°C, pH-6.5-7.5, and aeration 0.8-1.0 vvm. The duration of fermentation is around 4 days.
Recovery of chlortetracycline:
At the end of the fermentation, the culture broth is filtered to remove the mycelium. The filtrate is treated with n-butanol or methylisobutylketone in acidic or alkaline condition for extracting the antibiotic. It is then absorbed to activated charcoal to remove other impurities. Chlortetracycline is eluted and crystallized.
Production of Tetracycline —Different Processes:
The production of tetracycline can be achieved by one or more of the following ways.
i. By chemical treatment of chlortetracycline.
ii. By carrying out fermentation in a chloride-free culture medium.
iii. By employing mutants in which chlorination reaction does not occur.
iv. By blocking chlorination reaction by the addition of inhibitors e.g. thiourea, 2-thiouracil.
Type # 5. Macrolides:
Macrolides are a group of antibiotics with large lactone rings (i.e. macrocylic lactone rings). They consist of 12-, 14-, or 16-membered lactone rings with 1-3 sugars linked by glycosidic bonds. The sugars may be 6-deoxyhexoses or amino sugars. Erythromycin and oleandomycin are 14-membered (lactone ring containing) macrolides while leucomycin and tylosin are examples for 16-membered microlides.
Erythromycin and its derivative clarithromycin are the most commonly prescribed microlides. They are effective against Gram-positive bacteria, and are frequently used to kill penicillin-resistant organisms. Clarithromycin is currently used to combat stomach ulcers caused by H. pylori. The macrolides inhibit the protein biosynthesis by binding to 50S ribosome. Polyene macrolides is the term applied for very large ring macrolides that many contain lactone rings in the range of 26-28. e.g. nystatin, amphotericin. These polyene macrolides are antifungal.
Production of Macrolides:
Macrolides are produced by actinomycetes. The major macrolide antibiotics and the corresponding organisms synthesizing them are given in Table 25.3.
Biosynthesis of Erythromycin:
In the biosynthesis of erythromycin, the lactone rings are contributed by acetate, propionate or butyrate while the sugar units are derived from glucose. Macrolide biosynthesis is a complex process and a good example of polyketide synthesis which is analogous to fatty acid biosynthesis. The enzyme lactone synthase is a multi-enzyme complex which is comparable in its structure and function to fatty acid synthase complex. An outline of the biosynthesis of erythromycin is given in Fig. 25.13.
Regulation of biosynthesis:
End product inhibition of erythromycin synthesis is well documented. Erythronolide B inhibits the enzyme lactone synthase. The final product erythromycin has also been shown to inhibit certain enzymes of the pathway (e.g. transmethylase). Addition of propanol to the culture medium induces the synthesis of acetyl CoA carboxylase, and almost doubles the production of erythromycin.
Production Process of Erythromycin:
Industrial production of erythromycin is carried out by aerobic submerged fermentation. The culture medium mainly consists of soy meal or corn steep liquor, glucose (or starch), yeast extract and ammonium sulfate. Fermentation is carried out at 30-34°C for about 3-7 days. Conventional methods are used for the recovery and purification of erythromycin.
Type # 6. Aromatic Antibiotics:
The antibiotics with aromatic rings in their structure are regarded as aromatic antibiotics. In a strict since, all the antibiotics containing aromatic nuclei should be considered in this group. However, most authors prefer to treat the three important antibiotics namely chloramphenicol, griseofulvin (Fig. 25.14) and novobiocin in the category of aromatic antibiotics, and the same is done in this book also.
Chloramphenicol:
Chloramphenicol is a broad spectrum antibiotic that can act against Gram-positive and Gram- negative bacteria, besides rickettsia’s, actinomycetes and chlamydia’s. However, administration of chloramphenicol is associated with side effects, the most significant being damage to bone-marrow. As such, chloramphenicol is treated as a reserve antibiotic and selectively used. Chloramphenicol binds to 50S ribosomal subunit and blocks (peptidyltransferase reaction) protein biosynthesis.
Production of chloramphenicol:
Chloramphenicol can be produced by Streptomyces venezuelae and S. omiyanesis. However, chemical synthesis is mostly preferred for the commercial production of chloramphenicol.
Griseofulvin:
Griseofulvin is an antibiotic that acts specifically on fungi with chitinous cell walls. It is used in the treatment of various fungal skin infections. Further, griseofulvin is also employed in the treatment of plant diseases caused by Biotrytis and Alternaria solani. Although the exact mechanism of action of griseofulvin is not known, it is believed that chitin biosynthesis is adversely affected.
Production of griseofulvin:
Commercial production of griseofulvin is carried out by employing Penicillium patulum. The chemical synthesis is less frequently used due to high cost. The fermentation is carried out by an aerobic submerged process with a glucose rich medium. Nitrogen is supplied by sodium nitrate. The optimal conditions for fermentation are—temperature 23-26°C, pH 6.8-7.3, aeration 0.8-1 vvm, and the period is 7-10 days.
Type # 7. Nucleoside Antibiotics:
There are several antibiotics (more than 200 or so) which have nucleoside like structures e.g. puromycin,. blasticidin S. Nucleoside antibiotics have diverse structures and biological activities. Puromycin is used to understand the ribosomal function in protein biosynthesis. Neplanosin possesses antiviral activity. Blasticidin S is a fungicide antibiotic used in plant pathology.
Production of nucleoside antibiotics:
Selected examples of nucleoside antibiotics and the respective organisms from which they are produced are given below.
Puromycin — Streptomyces alboniger
Neplamosin A — Ampullariella regularis
Blasticidin S — S. griseochromogenes
Polyoxins — S. cacaoi
Genetic Manipulations of Steptomyces:
A great majority of antibiotics are produced by Streptomyces sp, a Gram-positive bacteria. Of course, there are some other bacteria (both Gram- positive and Gram-negative) and fungi that can also produce antibiotics. Genetic manipulations in Streptomyces have been extensively carried out to enhance the yield of antibiotics and reduce cost of production, besides developing newer and more effective antibiotics.
Transformation of Streptomyces:
Streptomyces strains exist as aggregates of mycelial filaments and not as individual cells. This is in contrast to E. coli. It is therefore essential that the cell walls of Streptomyces are broken to release protoplasts for transformation (Fig. 25.15). By adding the desired DNA (in plasmids) and polyethylene glycol, the cells can be transformed. These protoplasts are grown on a solid medium to regenerate the cell walls. The transformed cells with desired properties can be isolated for further use.
Cloning of Antibiotic Biosynthesis Genes:
In general, biosynthesis of antibiotics involves several reactions and participation of a large number of enzymes (and of course, several genes). It is rather difficult to clone so many genes. Mutant strains of Streptomyces that totally lack the synthesizing machinery for a particular antibiotic are very useful. Genes from a clone bank can be incorporated into such mutants and screened for the desired properties. This is a lengthy and tedious procedure but has been successfully used for the improved production of certain antibiotics e.g. undeylprodigiosin.
Direct strategies of gene cloning:
It is often possible to identify one or a few important enzymes in the synthesis of antibiotics. From the sequence of amino acids in the enzyme, gene can be constructed, and cloned. For instance, the gene for isopenicillin N synthase from P. chrysogenum has been successfully constructed in this fashion and used for increased production of penicillins and cephalosporin’s.
Genetic Engineering for the Production of Novel Antibiotics:
Genetic manipulations can be done in the antibiotic synthesizing organisms to ultimately produce totally new and novel antibiotics. Wild-type Streptomyces coelicolor genes encode the enzymes to produce the antibiotic actinorhodine. S. violaceoruber produces a related antibiotic namely granaticin. Genetic manipulations can be done between these organisms to produce novel hybrid antibiotics such as medarrhodine A and dihydrogranatirhodine.
The newly synthesized antibiotics are in fact structural variants of the existing antibiotics. As the biosynthetic pathways for antibiotic production and their corresponding genes are better understood, it becomes possible to design newer antibiotics with more efficient action.
Genetic Engineering for Improving Antibiotic Production:
For aerobic microorganisms (e.g. Streptomyces sp), there is often the limitation of oxygen supply that hinders antibiotic production. Some workers have isolated a hemoglobin-like protein produced by the bacterium Vitreoscilla sp. The gene synthesizing this protein was isolated and cloned in a plasmid vector.
The hemoglobin gene of Vitroscilla sp was finally incorporated into Streptomyces. These newly transformed strains have better capacity to take O2 from the medium even at a low concentration. The new strain of S. coelicolor (with hemoglobin gene) was found to produce 10 times more antibiotic actinorhodine than the wild strain, even at a low concentration of oxygen.
Good Antibiotic Manufacturing Practices:
Manufacture of antibiotics is highly commercialized due to a heavy demand worldwide. It is mandatory that detailed clinical trials are carried out before considering the manufacture of any antibiotic. More than half of the antibiotics produced are for human use. If is therefore absolutely essential that each antibiotic produced is safe, consistent and poses no health complications.
Government authorities play a predominant role in regulating the production of antibiotics. These guidelines ensure that correct procedures are followed at each stage of manufacturing the antibiotics. The product produced should be of consistently high quality. Quality of the products should be checked at different stages of manufacture.
It is expected that the antibiotic manufactured should be in the purest form, although 100% purity is not practicable. It is mandatory that the impurities (if any), their quantities, and their ill effects should be made known.