In this article we will discuss about Subphylum Urochordata:- 1. Important Features of Subphylum Urochordata 2. Fossil history of Subphylum Urochordata 3. Scheme of Classification 4. Colony Formation.
Important Features of Subphylum Urochordata:
The subphylum Urochordata constitutes a unique group of animals under the, Phylum Chordata. The members of this subphylum exhibit a high degree of diversity in form, habit and habitat.
1. AII urochordates are marine, sessile (Ascidiacea) or permanently pelagic (Larvacea or thaliacea) forms.
2. The adults are mostly degenerated and un-segmented.
3. The notochord is confined to the tail region in the larval stage. For this characteristic feature, the name of the group is given Urochordata. Other features — a dorsal hollow nerve cord, pharyngeal slits and a post-anal tail are found in the larval stage. They retain only pharyngeal (branchial) slits in the adult.
4. The body is enveloped by flexible tunic or test. Hence commonly known as tunicates. The material of the tunic is unique because it contains cellulose, a plant materials.
5. The free end of the body bears two openings — the mouth and the atriopore.
6. The proximal part of the alimentary canal is greatly enlarged to form a spacious pharynx, adapted for filter feeding.
7. The coelom is not recognisable.
8. Respiration performs by gaseous exchange across the tunic or the pharyngeal slits.
9. Blood vascular system is open. Heart is simple, tubular and ventral, enclosed in a pericardial cavity. The heart is unusual because it periodically reverses the direction in which it pumps blood. Blood contains cells but not erythrocytes.
10. Excretion is performed by neural gland, pyloric gland and nephrocytes.
11. The urochordates are mostly hermaphrodite, but in some species of Distaplia, Holozoa and Sycozoa (Ascidiacea) and in Oikopleura dioica (Larvacea), the uni-sexuality is noticed. The eggs and sperm attain maturity at different times in most species.
12. Fertilization is cross and external and takes place in sea water.
13. Asexual reproduction accomplishes by the formation of buds.
14. Development occurs through a free- swimming tadpole larva.
15. The urochordates exhibit certain exceptions to the characters of the chordates, like retrogressive metamorphosis, alternation of generations and the formation of colonies.
Fossil history of Subphylum Urochordata:
Jaekelocarpus oklahomensis recorded from the upper Carboniferous, Gene Autry Shale Formation near Ardmore of Oklahoma, U.S.A. is considered as fossil tunicate, belonged to the stem group of Urochordata, because it possessed calcite skeleton, ciliated pharyngeal slits and a downward flexing tail.
The pharyngeal slits have been suggested to be respiratory in function.
Size:
0.25 mm to 250 mm.
Distribution:
Urochordates are all marine, found in all seas from the intertidal zone to deep waters.
Habit and Habitat:
About most of the urochordates (95%) lead a free moving larval stage but in adults they lead to sessile (attached) and sedentary life. This type of life cycle is seen among ascidians, but larvaceans and thaliaceans lead throughout planktonic life. Most of the urochordates are sac-like creatures inhabiting the sea-bottom and are popularly called ‘sea-squirts’.
Scheme of Classification of Subphylum Urochordata:
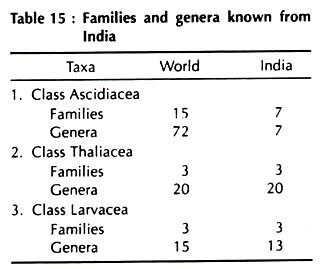
Berrill (1950), Marshall and Williams (1964), Ruppert and Barres (1994), Anderson (1998) and Pechenik (2000) have divided the Urochordata into different Classes and orders. Barrington (1967, 1979), Young (1981), Kent and Miller (1997) and Kardong (1998, 2002) have divided the Urochordata into three classes only (Ascidiacea, Thaliacea and Larvacea) and these classes do not pertain any order.
The outline classification of Urochordata according to Berrill (1950), Young (1981), Ruppert and Barnes (1994), Anderson (1998) and Pechenik (2000) has been given here in summarised form.
We follow in this text book Marshall and Wiliam’s (1964) scheme of classification.
Classification in Outline:
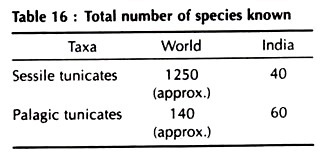
Subphylum. Urochordata Herdman, 1910 or Tunicata Lamarck, 1816 [Gk. Oura, the tail, or L. Tunicatus, clothed with the tunic]. Approx. – 1400 species.
Class 1. Ascidiacea
Order 1. Enterogona,
Suborder 1. Aplousobranchiata
Family 1. Clavelinidae
e.g., Polycitor, Tetrazona, Clavelina, Holozoa, Sigillina
Family 2. Polyclinidae
e.g., Polyclinum, Aplidium
Family 3. Didemnidae
e.g., Didemnum, Diplosoma
Suborder 2. Phlebobranchiata
(= Dictyobranchiata)
Family 1. Clionidae
e.g., Cliona
Family 2. Diazonidae
e.g., Diazona
Family 3. Perophoridae
e.g., Perophora
Family 4. Corellidae
e.g., Corella, Rhodosoma
Family 5. Ascidiidae
e.g., Ascidia, Ascidiella
Family 6. Hypobythiidae
e.g., Hypobythius
Order 2. Pleurogona
Family 1. Stylidae
e.g., Styela, Polycarpa Botryllus, Herdmania Peloneia
Family 2. Pyuridae
e.g., Pyura, Tethyum
Family 3. Molgulidae
e.g., Molgula, Eugyra, Anurella
Class 2.Thaliacea
Order 1. Doliolida (Cyclomyaria)
Family 1. Doliolidae
Doliolum, Doliopsis (= Anchi- nia), Dolioloides, Dolioletta
Order 2. Pyrosomida
e.g., Pyrosoma
Order 3. Salpida (Hemimyaria, Hetero- myaria)
Family Salpidae
e.g., Salpa, Cyclosalpa, Pegea, Thalia, Metcalfina, Jasis, Thetys, lasis.
Class 3. Larvacea or Appendicularia
Family 1. Kowalevskiidae
e.g., Kowalevskia
Family 2. Fritillaridae
e.g., Fritillaria, Appendicularia
Family 3. Oikopleuridae
e.g., Oikopleura, Folia, Megalocercus, Stegosoma, Pegalopleura.
Systematic Resume of the Urochordata:
The Subphylum Urochordata includes a large number of species which exhibit high degree of biological diversities. The members are classified under three classes: Ascidiacea, Thaliacea and Larvacea or Appendicularia.
Class Ascidiacea [Gk. askidion, diminutive of askos, a bag or bladder], Approx. 2,000 species.
i. Ascidians are marine and benthic forms which vary greatly in size and form.
ii. The individuals are solitary or colonial. The colonial animals are social and compound, and colonies are formed comprising a few to hundreds zooids.
iii. Adults become usually sessile after retrogressive metamorphosis of the larval stage, when the notochord, nerve cord and tail are lost.
iv. The outer surface of the body is covered by a permanent, thick cuticular covering called test or tunic, composed of a special protein, the tunicin and polysaccharides related to plant cellulose.
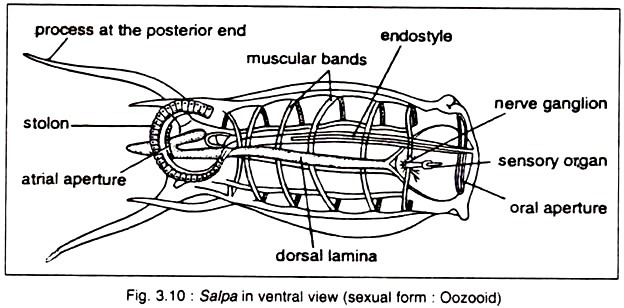
v. Animals are fixed to the substratum by one end, and the other free end bears two openings — oral or branchial aperture at the top and the atrial aperture at the side. The stream of water enters through the oral or branchial opening and exits through the atrial siphon.
vi. The pharynx or branchial basket is unusually large with a persistent few to numerous ciliated slits, called stigmata. The pharynx is used for filter feeding.
vii. The intestine is U-shaped. Digestion is extracellular.
viii. There is well-developed blood vascular system including a tubular heart, covered by pericardium. Circulation is through a system of channels, called haemocoel. The blood contains various pigments but these pigments are non-respiratory in nature. The system is most peculiar for the periodic reversal of the flow through the circuit.
ix. Nervous system is simple consisting of a cerebral ganglion or brain which is situated in the connective tissue between the two siphons.
x. Special sensory organs are lacking.
xi. Mostly hermaphrodite but in a species of some genera (e.g., Distaplia, Holozoa, Sycozoa), the uni-sexuality is observed.
xii. Fertilization takes place externally in sea water or within the atrium.
xiii. Mostly solitary ascidians. are oviparous and most compound ascidians are ovoviviparous and a few species of the families of Molgulidae and Styelidae develop directly.
xiv. Eggs are yolky and develop into lecithotrophic, planktonic larvae.
xv. The larva is free-swimming with a bulging trunk and long tail, resembles the shape of a tadpole, hence, called tadpole larva.
xvi. Colonial ascidians are produced by asexual budding.
They are commonly called “sea squirts”, because the solitary forms spray water when they are disturbed mechanically.
Habit and Habitat:
They are fixed to the hard rocks, stones, on the undersurface of boats, ships, jetties, on the barnacles, pearl oyster beds, mangrove roots and on floating objects. They are also attached to the muddy and sandy substratum and some are interstitial.
They are unable to survive in low salinity areas except Cnemidocarpa zenkevitchi which is found in the brackish water bays off the knox coast in the Antarctic.
Geographical distribution:
Ascidians are exclusively marine, found in all seas extending from the tropical to polar regions. They occur in both shallow and deep water. Deep water forms live in between the depth of 2000 m to about 8000 m.
Order Enterogona:
The body may be divided into two regions. The neural gland is usually situated ventral to the nerve ganglion. Single gonad is present which is located in or behind the intestinal loop. The larva possesses two sense- organs. Examples: Ascidia, Ciona, Clavelina, Phallusia.
Order Pleurogona:
The body is undivided. The neural gland is usually located either laterally or dorsal to the nerve ganglion. There are two gonads located in the lateral wall of the mantle. The larva bears only one sense organ.
Examples: Molgula, Botryllus, Styela, Amaroucium, Herdmania.
Molgula is a very important European monoascidian (Fig. 3.8A). It is built on the typical ascidian plan. The body is either ovoid or spherical in shape and measures about 3-8 cm in diameter. Both the oral and atrial funnels are large and are produced into six lobes. The test contains fibrils and sand grains adhered to the surface of the test. The stigmata are spiral.
The internal cavity of the pharynx usually shows seven longitudinal folds. A single large renal sac is present in the right side of the body. Two club-shaped gonads, one on each side of the body, are present. Molgula is oviparous and the development is either direct or indirect.
In some species of the genus like Molgula arenosa, Molgula occulta, Molgula manhattensis—the development occurs without any larval stage, while others are larviparous.
Botryllus is a compound ascidian in which the individuals are embedded in a common gelatinous test (Fig. 3.8B). The colony is star- shaped and remains attached to the hard surface of the bottom.
The individuals are arranged in a radiating manner around a common cloacal chamber. Each zooid has a separate oral funnel at the ends of the radii but their atrial apertures lead into a common cloacal chamber situated at the centre of the colony. Each individual is built on the typical ascidian plan.
Class Thaliacea [Gk. thalia, a muse]. Approx. 70 species:
This class includes the free-swimming pelagic tunicates.
i. The members of this class vary greatly in size. The adult form is devoid of notochord and tail.
ii. The tunic is a permanent structure which bears circular muscle bands.
iii. The tunic is thin and transparent.
iv. Oral and atrial siphons are situated at opposite ends of the body.
v. The pharynx is provided with two large or many small stigmata.
vi. Distinct alternation of generations is present in the life-cycle.
Geographical distribution:
Tropical and subtropical seas. Several species have recorded from colder waters. All members except one (e.g., Oikopleura dioica) are monoecious and most of these are protandrous.
Order Doliolida (Cyclomyaria):
The body is barrel-shaped with complete muscle bands forming eight regular rings. The number of gill-slits are variable. Usually a tailed larval stage is present in the life-cycle.
Examples:
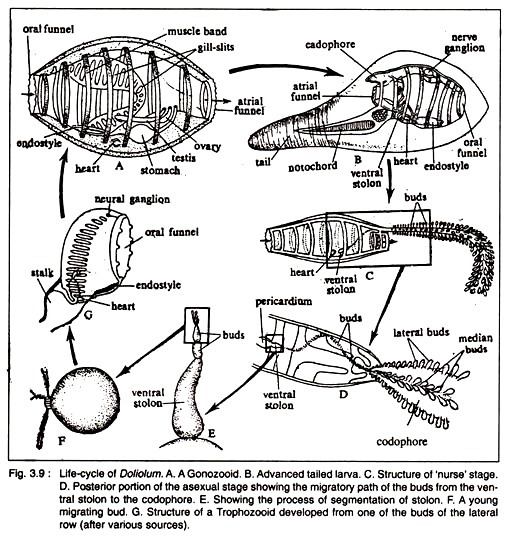
Doliolum (Fig. 3.9A) and Dolichinia.
The anatomical organisation of Doliolum is basically similar to other ascidians but it differs in certain points. The solitary sexual form (Gonozooid) is barrel-shaped and is provided with seven or eight muscle bands that encircle the body completely (Cyclomyaria).
By the contraction of these muscle bands, Doliolum swims in water. The oral and atrial funnels become elaborated into ten to twelve lobes at the two extremities of the body (Fig. 3.9A).
The test is very thin, transparent and non-cellular. The oral funnel opens into a spacious pharynx, the wall of which is perforated by the dorsal and ventral rows of stigmata. The dorsal lamma is absent, but the endostyle and the peripharyngeal ciliated bands are present. The oesophagus leads into a small stomach. The intestine is very short and is partially U shaped.
The dorsal side is comparatively extensive. The dorsal neural ganglion and sub- neural gland are located anteriorly. The neural ganglion gives nerves to the eight muscle bands. The heart is located posterior to the endostyle.
The sexes are united. The free- swimming Doliolum has originated from sessile ancestor. The ovary and testis are located ventral to the body. The eggs are liberated in the cloacal chamber and are finally expelled to the sea water. Fertilization is external.
Development and life-history:
Cleavage is holoblastic. Gastrulation occurs in embolic fashion. A tailed tadpole larva emerges after gastrulation. The tailed larva represents the asexual stage (Fig. 3.9B). The tail is comparatively short and acts as the locomotors organ. Metamorphosis of the tadpole larva starts before the larva becomes fixed to the substratum.
The process of metamorphosis is shown in Fig. 3.9. The tail is gradually lost and the tadpole larva is transformed into a ‘Nurse Zooid’ or ‘Oozooid’ having a cadophore on the posterodorsal side and a ventral stolon. Numerous buds are developed on the posterodorsal side and a ventral stolon. The buds have the power of migration and they become localised on the cadophore in rows.
The cadophore becomes elongated and most of the buds transform into zooids. The zooids present on the cadophore are divided into two major types—the lateral and median zooids. The lateral zooids are converted into the trophozooids.
But some of the median zooids develop into the phorozooids. On the stalk of the phorozooids a few buds remain attached which ultimately develop into the gonozooids. So in the life of Doliolum, there are three types of zooids.
The biology of the zooids are:
(1) Trophozooids:
These sessile zooids are nutritive and respiratory in functions. These are sterile and remain attached with parent.
(2) Phorozooids:
These are also sterile, but are set free in water.
(3) Gonozooids:
The gonozooids are sexual forms, which are carried and nursed by the phorozooids until sexual maturity is attained. After attaining sexual maturity, the gonozooids become detached from the phorozooids and lead independent free-swimming life.
Order Pyrosomida:
This order includes only one species, Pyro soma. It is present in all oceans—from the surface to a considerable depth. It forms a compact tubular colony. The zooids are embedded in the wall. The muscle bands are present at the end of the body. There is no larval stage in the life history. It reproduces by budding.
Pyro soma is colonial pelagic tunicate (Fig. 3.8C). The colony consists of many zooids or individuals which are associated together to form an elongated hollow colony. One end of the colony is closed and the other end is open. The open end serves as the common ‘cloaca’ for all the zooids. This aperture of the colony is guarded by a velum (Fig. 3.8D).
The opening and closure of the aperture are controlled by the velum and thus regulate the water flow. The zooids are arranged in an organised fashion in the wall of the colony. The oral ends open to the outside and the atriopores open into the hollow of the colony (Fig. 3.8E). The zooids are of asexual types and are capable of budding. The colony is brilliantly phosphorescent.
The light is emitted by the photogenic organs situated on each side of the pharynx. These organs are composed of photogenic cells. The photogenic cells contain numerous curved inclusions in the cytoplasm.
Actual nature of such inclusions is not known although claims have been made by some workers that these are possibly some forms of symbiotic luminescent bacteria. The light is not produced at all the times, but only when the animals are stimulated even by the waves of a rough sea.
Order Salpida (Hemimyaria, Heteromyaria):
The body is cylindrical. The muscle bands are incomplete, i.e., the muscle bands do not form complete rings as seen in Doliolum. It is present in coastal to deeper zone of 4,500 feet depth. The first gill-slit forms a large opening in adults. The larval stage is lacking.
Example:
Salpa (Fig. 3.10).
The life-history of Salpa involves alternation of generations. The sexual form produces a single egg which develops within the mother and is nourished by a diffused placenta. The tadpole larva is absent. The developing embryo becomes the ‘Nurse Zooid’ (Fig. 3.10) or ‘Oozooid’ which produces a long chain-like blastozooids. The blastozooids are set free in course of time.
Class Larvacea or Appendicularia (Gk. Larva, the immature form of a changing animal]. Approx. 70 species:
i. The members of the class are minute free-swimming pelagic forms with a highly developed tail.
ii. The tail is supported by notochord and large striped muscle cells.
iii. The body is about 5 mm long.
iv. The tunic is not persistent, instead each zooid builds a ‘house’ secreted by the skin.
v. The nerve cord is persistent.
vi. The pharynx bears two stigmata which open directly to the exterior.
vii. The atrium is lacking.
Examples:
Oikopleura (Fig. 3.11 A) and Appendicularia (Fig. 3.11 B) .
All members except one (e.g., Oikopleura dioica) are monoecious and most of these are protandrous.
Oikopleura is a tiny, neotenous free- swimming and pelagic tunicate (Fig. 3.11 A). It has a rounded body and a tail-like appendage attached to the posterior end of the body. The tail is supported by the notochord and also contains the nerve cord and muscles.
The muscles do not exhibit metamerism. The tail has a wide and continuous fin. The tail is an efficient locomotors organ and remains attached with the body by a ball-and-socket mechanism.
The test is absent and each individual makes a ‘house’ out of its test to envelope itself. The house is secreted by the ‘oikoplastic epithelium’—a special part of the skin. A constant water current is maintained by the activity of the tail. The water current enters into the house by a pair of filtering windows on the posterior side and passes out through a system of filtering pipes in front of the mouth (Fig. 3.11 A).
Minute ciliates or flagellates are caught in the filtering pipes and are sucked by the mouth. The pharynx bears two gill-slits — endostyle and peripharyngeal bands. The alimentary canal is U-shaped. A large hermaphroditic gland is present in the posterior end of the trunk.
Appendicularians are neotenous, minute, free-swimming pelagic solitary forms (Fig. 3.11 B). A gelatinous transparent covering, the house, contains the body proper. It is loosely attached to the body and not made of tunicin. Its function is protective and to some extent hydrostatic.
The length of the body is about 2 mm. The laterally compressed long tail is attached to the ventral surface of the body, serving for locomotion, nutrition and also in the building of the house. The tail has a continuous fin and supported by the notochord. 10 large striped bands run from the anterior to posterior region. The pharynx has two gill-slits and the endostyle is straight.
There is no separate atrial or cloacal opening. The anus opens ventrally on the surface of the body usually in front of stigmata. Metamorphosis does not take place in the life-history. They occur near the surface of the sea in most of the parts of the world.
Geographical distribution:
They occur as plankton in all seas, particularly common in shallow coastal waters. A few are found at great depths.
Colony Formation in Urochordates:
Colony formation in some urochordates is a very peculiar occurrence. The colony is formed by the budding off of non-separable individuals or zooids from the parent. Ascidians do not form true colonies. The members of the order Enterogona usually bud off new individuals which are complete by themselves. They may be mechanically connected with the parent body.
Some members of the order Pleurogona have individuals that are buried in common investing mass to form a colony. In Botryllus, a few individuals remain embedded in a common tunic. They have separate oral apertures, but their atrial apertures open into a common cloacal chamber.
The most peculiar type of colonial urochordate is Pyrosoma. The individuals form a hollow cylinder. The cylinder is closed at one end and the other end is open. This opening is guarded by velum which acts as the regulator of the water flow. The oral funnels of the individuals are situated on the outer side and the atrial funnels open into the cavity of the cylinder.
All the zooids originate from a single sexual progenitor by budding. The budded individuals do not separate themselves from the parent body but remain organically connected with one another. Such a phenomenon leads to the formation of colony, a feature not ordinarily encountered in chordates.