In this article we will discuss about Branchiostoma:- 1. Geographical Distribution of Branchiostoma 2. Habit and Habitat of Branchiostoma 3. External Structures 4. Body Wall 5. Supporting Structures 6. Locomotion 7. Digestive and Respiratory Systems 8. Mechanism of Feeding and Digestion 9. Circulatory System 10. Excretory System 11. Nervous System 12. Receptor Organs 13. Reproductive System and Other Details.
Contents:
- Geographical Distribution of Branchiostoma
- Habit and Habitat of Branchiostoma
- External Structures of Branchiostoma
- Body Wall of Branchiostoma
- Supporting Structures of Branchiostoma
- Locomotion in Branchiostoma
- Digestive and Respiratory Systems of Branchiostoma
- Mechanism of Feeding and Digestion in Branchiostoma
- Circulatory System of Branchiostoma
- Excretory System of Branchiostoma
- Nervous System of Branchiostoma
- Receptor Organs in Branchiostoma
- Reproductive System of Branchiostoma
- Development of Branchiostoma
- Primitive, Degenerate and Specialized Features of Branchiostoma
- Affinities and Systematic Position of Branchiostoma
1. Geographical Distribution of Branchiostoma:
It has almost a cosmopolitan distribution and found on the sandy shores covering the tidal area to depths of many meters. It is an inhabitant of the shores of tropical and temperate seas. The common lancelet, Branchiostoma lanceolatum, has been recorded from the West and Southern European coasts, on the East African coasts and also from the western and south-eastern Indian coasts.
2. Habit and Habitat of Branchiostoma:
Branchiostoma live both in marine and estuarine habitats. It is commonly found in the sandy shores. It lives in dilute sea-water between 15.4% and 33.1% salinities. Different species may show different degrees of tolerance to salinity changes.
Webb and Hill (1958) reported that the lower threshold salinity for adult B. nigeriense is 13%. B. lanceolatum is mostly marine in its distribution and stenohaline (animals restricted to a narrow range of environmental salt concentrations) in its behaviour. The limits of salinity tolerance in sea-water are 34%.
It leads a dual life. It is a sedentary animal although it can swim actively in water. It swims vertically in water. Branchiostoma can swim in water like a fish. Swimming is done by a wave of contraction originating from the anterior end of the body and passing down the longitudinal muscles. During this process each myotome contracts after its anterior one.
The wave of contraction passes alternately along the left and right sides of the body. Such waves produce a side-to-side bending of the body for forward propulsion. The animal swims spirally about its axis. The notochord with its limited elasticity acts as the lever upon which the muscles work. The notochord also resists shortening of the body during muscular contraction.
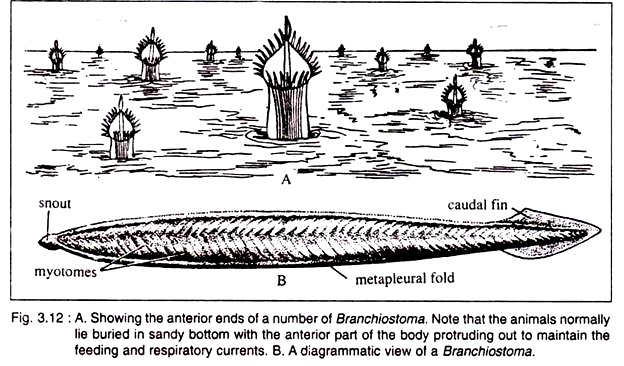
The fins do not play any role during swimming. It burrows in the sand, but remains always at a small depth. It keeps the anterior end of the body projected above the sandy bed to maintain a constant water current passing in through the mouth and expelled through the atriopore (Fig. 3.12A).
Branchiostoma feeds on micro-organisms brought into the pharyngeal cavity together with the respiratory water current. It exhibits a typical instance of ciliary mode of feeding.
3. External Structures of Branchiostoma:
Branchiostoma is a small lancet-shaped creature with sharply pointed anterior and posterior ends (Fig. 3.12B). The length of the animal varies from 5-8 cm. The body is elongated and flattened from side to side. The body is distinguishable into the body proper and definite postanal tail. The pointed anterior end projects forward as snout or rostrum.
Below the snout lies the oral hood formed by dorsal and lateral projections of the body. The oral hood bears more than twenty stiff buccal or oral cirri or tentacles. The oral hood is supported internally by a series of gelatinous but stiff pieces arranged end to end.
Each oral cirrus is supported internally by stiff rod that emerges from this oral ring. The mouth is kept hidden within the oral hood. The anus is situated on the left side of the ventral fin and at a short distance cephalad from the posterior end of the body.
The atrium opens to the exterior through a round atriopore located close to the anterior end of the ventral fin. The anterior portion of the body is roughly triangular in outline while the posterior part behind the atriopore is more or less oval in cross-section. On the lateral sides of the body proper there are numerous gill-slits which remain partly covered by the lateral folds of the body. As a result the gill-slits are not ordinarily seen from outside.
The body is provided with a median longitudinal fold, called dorsal fin which extends along the whole of the dorsal side. The dorsal fin is joined to a somewhat broader caudal fin present round the tail. A ventral fin runs along the mid-ventral line lying between caudal fin and atriopore. The dorsal and ventral fins are supported by a series of connective tissues called fin-ray boxes.
Running along the ventrolateral sides of the anterior two-third of the body are two longitudinal folds, called meta-pleural folds. These meta-pleural folds extend between the oral hood and the atriopore. The meta-pleural folds do not participate in the process of swimming.
They possibly help the animal during burrowing in the sand. During the process of burrowing the meta-pleural folds become distended with the contained lymph in their cavities and thus assist in rapid drives into the sand.
The paired fins are absent but the meta-pleural folds are often homologized with lateral fin folds, from which the vertebrate limbs might have evolved. But this idea is now rejected because Branchiostoma is removed at present from the main line of vertebrate evolution.
4. Body Wall of Branchiostoma:
The epidermis consists of a single layer of cells. The epithelial cells are of columnar type (Fig. 3.13). These cells are ciliated in the early stage and in the adult the outer margin of the cells become cuticularised. The chemical nature of the cuticle is not known. The epithelial layer contains cells with sensory hairs (receptor cells), but gland cells and pigment cells are absent.
Immediately beneath the epidermis, there are two layers—cutis and sub-cutis. Both these layers are secreted by some scattered distributed wandering cells akin to the fibroblasts of the vertebrates. The cutis is made up of fibres and the sub-cutis has a gelatinous matrix containing fibres. A system of canals traverse through both the cutis and sub-cutis.
Beneath the sub-cutis lies the characteristic muscle layer of Branchiostoma. The mesodermal layer applied to the gut (splanchnopleure) becomes greatly thickened near the notochord to transform into the myotomes (Fig. 3.12) or myomeres.
Besides the myotomes, other muscles are also developed from the splanchnopleure and somatopleure (the mesodermal layer applied to the body wall). The myotomes are blocks of striated muscle fibres extending through the entire length of the body.
The myotomes are separated from one another by dense connective tissue septa, called myocommas. The myocommas are V- shaped and the pointed end of the ‘V’ is directed towards the anterior end. Although the myocommas are V-shaped, the muscle fibres composing the myotomes run straight, i.e., arranged longitudinally.
Each muscle fibre is attached to two successive myocommas. The myotomes are so arranged that the body can be twisted sidewise with considerable rapidity. The myotomes on the two sides of the body alternate with one another. Such an arrangement of myotomes helps lateral undulation of the body during locomotion. There are about 60 pairs of myotomes in Branchiostoma.
The most significant point in the muscular system of Branchiostoma is the great thickness of the musculature of the dorsal body wall. In non-chordates the muscle layer is of almost uniform thickness surrounding the coelom.
5. Supporting Structures of Branchiostoma:
The notochord extends from the tip of the head to tail end. Such anterior prolongation of the notochord beyond the brain is associated with its burrowing habit. This chord is situated on the mid-dorsal line above the alimentary canal. It is composed of a series of flat plates arranged along the transverse plane of the body.
Two kinds of plates alternate with each other, one is fibrous in nature and the other is homogeneous. The plates are enveloped by an outer fibrous and an inner elastic notochordal sheath. Each plate is derived from a single cell which becomes highly vacuolated and the nucleus is pushed to one side.
Surrounding the notochordal sheath there is a non-cellular gelatinous layer containing few fibres. The other supporting structures include the gill-rods supporting the gill-bars, the skeletal oral ring supporting the oral hood and cirri and the fin-ray boxes supporting the dorsal and ventral fins (Fig. 3.14A).
6. Locomotion in Branchiostoma:
Branchiostoma is a sluggish animal by nature and it occasionally swims freely in water (when it is disturbed) by contraction of the longitudinal muscle fibres of the myotomes. Contraction of the myotomes causes the transverse motion of the body at different angles in such a fashion that the animal can move forward.
All the myotomes contract rhythmically along the anteroposterior direction, i.e., a myotome contracts after that in front of it.
During forward propulsion the myotomes contract to produce waves of contraction from anterior to the posterior side through the water. The myotomes are located on the lateral side of the notochord which forms the supporting structure. The function of the notochord is to prevent shortening of the body length when the muscle fibres of the myotomes contract.
The elasticity of the notochord helps to make the contraction of the body more efficient. The notochord acts as the lever upon which the myotomes work. The myotomes have no direct connection with the notochord, but the myocommas are attached with the notochordal sheath.
Branchiostoma cannot move very fast in water and the elaborate fin-system as seen in fishes is absent. The caudal fin is feebly developed. It normally resists transverse movements of the body and can accelerate transverse movements in emergency.
7. Digestive and Respiratory Systems of Branchiostoma:
The digestive system of Branchiostoma consists of an alimentary canal and digestive glands. The alimentary canal is again divided into mouth, oral hood and buccal cavity (vestibule), pharynx or branchial sac, oesophagus, intestine and anus. Hepatic diverticulum is often referred to as digestive gland.
A. The description of the different parts of the alimentary canal is given below:
Mouth:
Mouth is a small rounded aperture situated at the base of the vestibule or buccal cavity of the oral hood. It is surrounded by a membrane, called velum, acts as a sphincter which bears 12 slender velar tentacles or languets on its free edge, and help to close or open the mouth.
Oral hood and buccal cavity:
A large median aperture is situated just below the pointed tip of the anterior end (rostrum). This aperture is surrounded by a frill-like membrane, called oral hood (Fig. 3.14A). The membranous lateral and ventral margins of the oral hood are fringed with buccal cirri (Fig. 3.14A).
The buccal cirri are beset with sensory cells some of which are mechanoreceptors. Sensory cells on each cirrus probably provide the animal with information on water quality.
The oral hood and buccal cirri are supported by skeletal rods. During the inflow of water current, the buccal cirri form a sieve to prevent the entry of large particles. The oral hood encloses a cavity, called vestibule or buccal cavity. It is a short part of the foregut that receives water and food particles from the mouth.
Wheel organ:
The inner lining of the vestibule produces a complicated ciliated grooves and ridges, called wheel or rotatory organ or ciliated organ of Muller (Fig. 3.14A). The wheel organ produces a whirling currents of water to sweep the food matters into the mouth.
Hatschek’s groove:
There is a ciliated glandular groove running along the roof of the vestibule, called Hatschek’s groove. It is lined partly by endocrine cells which are believed to secrete pituitary-like hormones into the blood.
The groove of Hatschek terminates into a small depression, called the pit of Hatschek (Fig. 3.15A). The left embryonic coelomic cavity of the region becomes reduced to form the pit of Hatschek of the adult.
Velum:
There is a ring of finger-like sensory tentacles around the mouth, called velum (Fig. 3.14A) which separates vestibule (buccal cavity) from the pharynx.
Pharynx or branchial sac:
The pharynx is a large laterally compressed tube which occupies more than half of the total surface area of the body. The lateral wall of the pharynx is perforated by obliquely arranged vertical apertures — the gill-slits or branchial slits (Fig. 3.14A, B).
The number of gill-slits is about 180 pairs. The gill-slits open into an ‘U’ shaped special cavity, the atrium or peribranchial cavity, which surrounds the pharynx on all sides except the dorsal. The atrium is closed anteriorly but opens to the exterior through an aperture, the atriopore which is situated behind the level-of pharynx. The whole cavity of atrium is lined by epithelium of ectodermal origin.
The gill-slits are separated from one another by the portion of the walls of the body and the pharynx. These portions are called the gill- bars or branchial lamellae, i.e., the gill-bars are actually the vertical portions of the main body wall and the pharyngeal wall. Such a portion of the gill-bar which encloses the coelom is called primary gill-bar.
With the advancement of age, each primary gill-slit is divided into two by the downward growth of the tongue-bar. These resultant gill-bars are named the secondary gill- bars which lack coelom. The gill-bars are provided with cilia. The gill-bars contain supporting gill-rods. The gill-rods are of two types— the primary gill-rods and the secondary gill- rods.
The terminal end of the primary gill-rod is forked and those of the secondary gill-rods are simple, i.e., unforked. Depending on the presence of particular type of gill-rods, the gill-slits are also designated as the primary or secondary ones.
The primary branchial lamellae are connected by transverse skeletal rods, called the synapticulae. The internal wall of the pharynx is ciliated. Several ciliated tracts are present inside the pharynx.
Endostyle:
An endostyle is a ciliary and glandular groove present on the floor of the pharynx. The histological structure of endostyle of Branchiostoma (Fig. 3.15 C) similar to that of Ascidia in which the structure of the organ has been discussed in detail.
The endostyle consists of a few tracts of ciliated cells alternating with mucus-secreting glandular bands (Fig. 3.15 C). According to Barrington (1968), the endostyle of Branchiostoma produces the hormonal iodothyromines, not well present in tunicates.
He has observed the concentration of radioactive iodine in the glandular columns of the endostyle. The extract contains iodine and, on administration, accelerates the metamorphosis of tadpole larva.
Epipharyngeal or hyper pharyngeal groove:
A ciliated median groove is present on the dorsal side of the pharyngeal cavity, called epipharyngeal or hyper pharyngeal groove. The epipharyngeal groove joins with the anterior end of the endostyle by peripharyngeal ciliated tracts.
Oesophagus:
The pharynx opens into a short, narrow, ciliated tube, called oesophagus (Fig. 3.16B) that opens into the midgut. The pharynx and oesophagus constitute the foregut.
Intestine:
The intestine (gut) is a straight ciliated tube which can be divided into two regions — midgut and hindgut (Fig. 3.16B). The midgut includes hepatic diverticulum and iliocolonic ring.
At the junction of oesophagus and the midgut there is a large single un-branched out-pouching, lying at the right side of the pharynx, called hepatic diverticulum (Fig. 3.16A). The posterior part of the hepatic diverticulum, the midgut narrows into a short, ciliated wider section, called the iliocolonic ring (Fig. 3.16B). The tract of the intestine is provided with a weak musculature.
Anus:
The intestine proceeds posteriorly as a straight hindgut which opens through the anus.
B. Digestive gland:
Hepatic diverticulum, the so-called liver, referred to as digestive gland in Branchiostoma. It is a large blind out-pouching, develops at the junction of the oesophagus and the midgut and lies at the right side of the pharynx. The inner walls of the diverticulum, specially the dorsal and ventral walls, are beset with cilia.
It contains zymogen cells which produce digestive enzymes (a lipase and a protease) and are carried into the lumen of the midgut by ciliary activity. It is also a part where fat is deposited.
8. Mechanism of Feeding and Digestion in Branchiostoma:
Food:
Branchiostoma is a microphagous animal. The food or ‘sea soup’ consists of protozoans, algae, diatoms and other organic particles.
Feeding:
Branchiostoma obtains food by filtering the stream of waters that enters the pharyngeal cavity. The wheel organ produces a vortex. The buccal cirri become curved to form a sieve to prevent the entry of large particles. The sensory papillae in the buccal cirri and velar tentacles act as chemoreceptors and taste the nature of the food particles and also estimate the size of food particles.
If food particles are large in size or liable to cause toxicity, these are expelled by the forceful expulsion of the water from the pharyngeal cavity. The ingress of water into the pharyngeal cavity through the mouth is controlled by the velum.
The pharynx plays the most important role in food collection. The major portion of the water passes out into the atrium through the gill-slits. The cilia present on the gill-bars beat to drive the water out into the atrium and, thus, facilitate the inflow of fresh water current through the mouth.
The food particles, due to their own weight, begin to fall on the floor of the pharyngeal cavity and are entangled by the sticky secretion of the mucus-secreting cells of the endostyle.
The cilia in the endostyle and gill-bars beat to produce an upward current to push the mucus-entangled food particles towards the epipharyngeal groove. The cilia of the endostyle also beat to drive the food along the peripharyngeal-ciliated tracts to the epipharyngeal groove.
The food is pushed backwards by the backward beating of the cilia of the epipharyngeal groove (Fig. 3.16B). The secretion of the glandular cells of the endostyle transforms the boluses of mucus- entangled food particles into a cord-like structure, known as food cord.
The food cord from the pharynx passes through the oesophagus into the hepatic diverticulum and midgut where this food cord is subjected to the action of digestive enzymes secreted by the hepatic diverticulum. The food cord from the hepatic diverticulum is pushed backwards by the cilia present in its cavity. The mucus-entangled food cord is rotated by the ciliary action in the ileocolon ring.
Digestion in Branchiostoma is both intracellular as well as extracellular. The intracellular digestion takes place inside the hepatic diverticulum while the extracellular digestion occurs inside the midgut. The secretory cells of the hepatic diverticulum contain zymogen granules and they show phagocytosis, i.e., the cells are able to engulf the food particles from the food cord and digest the food as seen in Amoeba and Hydra.
The phenomenon of phagocytosis is attested by the fact that carmine particles, after ingestion into the diverticulum, are taken inside the cells. The digestive enzymes in Branchiostoma are amylase, lipase and protease. The digested food is absorbed in the hindgut and the undigested particles are expelled through the anus.
The controlling mechanism of the ciliary mode of feeding in Branchiostoma is not clearly known. The afferent and efferent nerve fibres in the atrium presumably play the important role in feeding. The rate of water current is largely controlled by the intensity of beating of cilia and also the degree of contraction or dilatation of the inhalant and exhalant apertures.
The different receptors present on the velum and the atrium taste the nature of water current. If the water current contains any toxic substance, the atriopore closes and the water is regurgitated by sudden contraction of the pterygial muscles which form the floor of the atrium. Bone (1979) has shown that after ingestion of sufficient food, the food collection is stopped until the food that has been taken in is digested.
Respiratory mechanism:
The wall of the pharynx is highly vascular. The water current entering into the pharyngeal cavity brings fresh oxygen dissolved in water. The gill-bars contain blood vessels with many lateral branches. The blood circulates so close to the surface that these blood vessels are able to absorb oxygen and give off carbon dioxide very efficiently.
Since the blood of Branchiostoma lacks any respiratory pigment and also occurs in lymph-spaces in the fins and meta-pleural folds, it is doubtful whether the pharynx has any role in oxygenation.
Many workers are doubtful about the respiratory role of pharynx and lay more emphasis on its role in food concentration. They advocated that a considerable part of required amount of oxygen is drawn through these superficial areas.
9. Circulatory System of Branchiostoma:
The circulatory system in Branchiostoma is well-developed (Fig. 3.17). The blood is colourless, i.e., it lacks any respiratory pigment. The blood is also devoid of cells (such as corpuscles or amoebocytes).
Since respiratory pigment is absent in Branchiostoma, the question of oxygen carriage remains unsolved. It is presumed that the tension of dissolved oxygen acquired by diffusion is sufficient to carry on the vital activities.
Oxygenation in the gill-bars is negativated by Orton (1913) by establishing the fact that the blood actually leaves the gill- bars less rich in oxygen than when it enters into them. Oxygenation actually takes place in the lacunae situated close to the integument, especially those present in the meta-pleural folds. The blood vessels, except the dorsal aorta, are without any lining.
The heart is absent in Branchiostoma and there is no pericardial cavity. The blood vessels are muscular and pulsatile in nature. The anatomy of the system shows like that of all higher chordates but histologically similar to the circulatory system of invertebrates. A description of the main vessels and their branches is given below.
Sinus venosus:
The blood from the different parts of the body is collected into a large sac called sinus venosus. The sinus venosus is situated below the posterior end of the pharynx.
Ventral aorta:
From the sinus venosus a large median artery arises which extends forward below the pharynx. This artery is named the ventral aorta or truncus arteriosus or endostylar artery. The ventral aorta gives the branchial vessels carrying blood to the gill- bars. The branchial vessel, at the base of each primary gill-bar, dilates to form a tiny expansion, called branchial bulb or bulbule (plural bulbilli).
Dorsal aorta:
From the gill-bars the blood is collected by the paired dorsal aortae, situated one on each dorsolateral side of the pharynx. These paired aortae join posteriorly to form an unpaired median dorsal aorta. This dorsal aorta extends posteriorly up to the tip of the tail as caudal artery. The paired dorsal aortae give small arterial vessels to the nephridia. These vessels form a net of minute vessels, called nephric glomerular sinus.
The paired and unpaired dorsal aortae have many branches which lead into the lacunae, called myocoel (the space between the myotomes and the body wall). The whole of the intestine and the hepatic diverticulum have extensive blood plexus. True capillary system is absent in Branchiostoma.
Sub-intestinal vein:
The blood from the tail region is collected by a caudal vein. It proceeds forward to join the sub-intestinal vein. The sub-intestinal vein collects blood from the intestinal plexus.
Hepatic portal system:
The sub intestinal vein proceeds anteriorly as the hepatic portal vein. It runs ventrally along the hepatic diverticulum where it breaks into a capillary network. From hepatic diverticulum blood is collected by a hepatic vein which runs along the dorsal side of the hepatic diverticulum and joins with the contractile sinus venosus.
Hepatic portal system, first found in Branchiostoma, is the precursor of the hepatic system of vertebrates.
Cardinal veins:
The blood from the ventrolateral sides of the body wall is collected by two pairs of cardinal veins — the anterior cardinals and the posterior cardinals. The anterior and posterior cardinals of each side unite to form a common cardinal or ductus Cuvieri which passes ventrally to join the sinus venosus.
The sinus venosus, ventral vessel, branchial bulbs, nephric glomerulae and sub-intestinal vein are contractile. The rate of contraction is very slow and occurs once in two minutes. The phenomenon of contraction is irregular and is not controlled by any coordinated system.
Course of circulation:
The course of circulation in Branchiostoma is as follows:
(a) The blood circulates from the posterior to the anterior end through the ventral vessel, sub-intestinal vein and the posterior cardinal veins, whereas
(b) The paired and unpaired dorsal aortae and the anterior cardinal veins drive blood from the anterior to the posterior direction.
10. Excretory System of Branchiostoma:
The excretory system of Branchiostoma lacks a kidney and is most peculiar for the organization of solenocytes which are comparable with those found in platyhelminthes, annelids and molluscs.
The excretory organs of Branchiostoma include the nephridia, brown funnels and cells of the atrial wall. The main excretory organs of the Branchiostoma are so-called nephridia. Goodrich (1902) gave a detailed description of the excretory organs of Branchiostoma.
Nephridia:
There are about 90 pairs segmental nephridia in Branchiostoma. The nephridia, called protonephridia, are situated on the dorsolateral wall of the pharynx. Each nephridium is a bent vesicular sac having one horizontal limb and one vertical limb. The nephridia are segmentally arranged. Each sac corresponds to each primary gill-bar and opens by a nephridiopore to the atrium.
A large number of elongated tubular flame cells or solenocytes derived from mesodermal cells, open into the vesicle (Fig. 3.18A). Each solenocyte (Podocyte) measures about 50 µm and has a long tubular stalk with a tiny balloon-like cell-body at the terminal end. The cell-body gives off a flagellum through the hollow stalk which helps in eliminating the waste products (Fig. 3.18B).
The solenocytes become associated with nephric glomerular sinus which separates the solenocytes from the coelomic epithelium. Electron microscopy has revealed that the flame cells are the modified coelomic epithelial cells.
The flame cells have similarity with the podocytes that line the renal capsule of vertebrates and the peculiar name of these cells has been given the cyrtopodocytes (Fig. 3.19). The basal part of the cells covering the glomerular blood vessels is joined by a slit membrane. The basement membrane is absent in between blood and coelomic spaces.
Excretion takes place through the wall of solenocytes by diffusion through the thin walls and the products pass down into the cavity of the vesicle through the tubular part. Colour particles which are injected into the blood stream are not excreted by the flame cells.
On the contrary Ruppert and Barnes (1994) say that the physiological significance of the nephridia is not known and the primary urine in the subchordal coelom is formed by the ultrafiltration of blood across the blood vessel and may then be directed into the tubule for the formation of final urine.
This urine, finally leaves the body through the atriopore. Kardong (2002) presumes that the exact role of solenocytes is not clear, but their arrangement between blood vessel and atrium helps in removing metabolic wastes from the blood and flushed away by the water stream that passes through the atrium and atriopore.
Phylogeny:
Due to the absence of basement membrane between blood and coelomic spaces which are found in the solenocytes of polychaetes, it is considered that the excretory organs possess both proto-nephridial and metanephridial characteristic features.
Nephridium of Hatschek:
Besides the nephridia, a tube called the nephridium of Hatschek is regarded to be excretory in function. It arises from the mouth and proceeds forward to the right side of the notochord. It is an ectodermal derivative and gets blood supply from the dorsal aorta. Blood from the nephridium of Hatschek is also returned to the dorsal aorta.
Miscellaneous excretory organs:
Besides the nephridia following structures also assist in excretion:
Brown funnels:
A pair of brown funnels are also claimed to play excretory role. These are blind sac-like bodies at the anterior end of the atrium and protrude into the epibranchial coelom. Although these are assigned to be excretory structures by many workers, these are possibly receptor organs.
Atrial wall:
Groups of cells in the atrial wall sub serve excretory function.
Gonads:
Inside the gonads, particularly in the testes, there are yellow masses containing uric acid. These masses are expelled along with the expulsion of gametes.
11. Nervous System of Branchiostoma:
The nervous system of Branchiostoma consists of a hollow dorsal nerve cord situated just above the notochord. The anterior part of the nerve tube is slightly dilated to form the brain and the posterior part remains as the spinal cord. The nerve tube gives paired nerves in each segment of the body.
The paired nerves are actually the dorsal and ventral nerve roots which remain separate (Fig. 3.20). The ventral nerve root lies opposite to the myotome and the dorsal nerve root passes out between the myotomes.
The nerves are non-myelinated, i.e., the nerves are not en-sheathed by a thick myelin sheath as seen in the nerves of vertebrates. The ventral root carries the motor fibres, but the dorsal root is mixed. It carries motor fibres for the nonmyotomic muscles and sensory fibres for the segment.
At the anterior end of the nerve tube, the neurocoel becomes enlarged to form a ventricle. The first two anterior dorsal nerves emerge from it, but the corresponding ventral nerves are lacking. These two nerves convey the impulses from the receptors of the oral hood and buccal cirri.
An infundibular organ is present on the ventral wall of the ventricle (Fig. 3.20). It consists of tall cells with strong cilia. This organ gives origin to Reissner’s fibre which proceeds posteriorly along the nerve tube. The Reissner’s fibre is comparable to that of the vertebrates.
The cells of the infundibular organ contain neurosecretory material as observed in the fibres of the vertebrate hypophysial organ. In the young stage, the ventricle opens through a neuropore which becomes closed in the adult. The region of the closure is marked by a depression, called Kolliker’s pit.
The anterior end of the ventricle contains pigmented cells and sensory cells. Though this organ is regarded by many as photoreceptors, the photoreceptive function of these cells has not yet been experimentally proved.
The photo-sensory cells present on the spinal cord are actually the photoreceptors. This is quite evident from the experiment conducted by Parker. A beam of light will initiate movements of Branchiostoma only when the light falls on the body and not when directed on the head.
The spinal cord has a narrow central lumen and the orientation of “grey matter” and “white matter” is similar to that of vertebrates. The “grey matter”, i.e., the cell layer, is present surrounding the canal and the “white matter”, i.e., the fibrous layer, is situated on the outer side.
Scattered in the spinal cord there are photo-sensory cells (eye-spots) enclosed by a cup of pigment granules, the cells of Joseph and Hesse and the giant cells of Rohde (Fig. 3.20).
The cells of Hesse are distributed along the inner side of the tube along the entire length, whereas the cells of Joseph are present anterodorsally. The giant cells of Rohde have many dendrites and one axon. The axon of the anterior giant cells proceeds backward and that of the posterior ones run forward. The giant cells are absent between 13-39 segments.
12. Receptor Organs in Branchiostoma:
There are various types of receptor organs in Branchiostoma.
Some of the sense organs are:
a. Pigment spot:
There is an unpaired pigment spot on the anterior wall of the brain. This spot is usually referred to as cerebral eye which lacks lens and other structures. It is not photosensitive in nature.
b. Eye-spots:
Photosensitive cells enclosed by a cup of pigment granules (eyespots) are distributed on the spinal cord and remain oriented in different directions. These are photoreceptors.
c. Kolliker’s pit:
A ciliated depression at the anterior end of the brain is called Kolliker’s pit. In all probability this is a chemoreceptor. In larval stage its cavity remains in direct communication with the ventricle through the neuropore.
d. Sensory papillae:
The oral cirri and the velar tentacles are beset with modified sensory papillae which act as the chemoreceptors and tactile receptors.
e. Infundibular organs:
The infundibular organ, located at the floor of the ventricle, probably acts as photoreceptor.
f. Epidermal sensory cells:
Sensory cells are present on the surface of the body, especially on the dorsal side.
13. Reproductive System of Branchiostoma:
The sexes are separate in Branchiostoma. The gonads are simple pouch-like segmental organs situated in the ventro-lateral sides of the pharyngeal region between the 10th and 30th segments. The gonads are proliferated from the mesodermal cells and the gametes are developed from the walls of the gonads. It is claimed that each gonad is developed from a single cell.
The gonoducts are absent and the gametes are discharged into the atrium by dehiscence. From the atrium the gametes escape to the exterior through the atriopore along with the water current. Fertilization and development occur in sea-water. The eggs are small (microlecithal, i.e., the amount of yolk is very small), and isolecithal, i.e., yolk is distributed uniformly in the egg.
14. Development of Branchiostoma:
The development of Branchiostoma is shown in Figure 3.21 and the process of development has been described by different authors.
Cleavage:
The cleavage is holoblastic, i.e., the cleavage furrows divide the zygote completely. The holoblastic cleavage may be equal (when the resultant blastomeres are equal in size) or unequal (when the resultant blastomeres are unequal in size). It has been worked out by Hatschek (1882, 1892), Mac Bride (1898, 1909) and Conclin (1932, 1933). Further the embryonic development of Branchiostoma is of indeterminate type.
The first cleavage is meridional, i.e., the cleavage furrow bisects the egg along the median axis or centre. The second cleavage is also meridional but passes at right angle to the first one.
Four equal-sized blastomeres are produced. The third cleavage is latitudinal and occurs slightly above the equatorial plane resulting in the production of eight blastomeres—four smaller ones are called micromeres and four larger ones are known as macromeres.
The micromeres form the animal pole while the macromeres form the vegetal pole. The fourth cleavage is meridional which involves all the eight cells resulting in the formation of eight micromeres and eight macromeres. The fifth cleavage planes are latitudinal. Each micromere is divided into an upper and a lower macromere. The fifth cleavage plane produces thirty-two blastomeres.
The sixth cleavage planes are nearly meridional involving all the thirty-two blastomeres resulting in sixty-four cells. At the sixty-four cell stage a conspicuous space is produced at the centre and this space becomes filled with a fluid.
During eighth cleavage stage, the blastula becomes pear-shaped and the blastocoel inside it becomes large. The blastula of Branchiostoma is called coeloblastula. The roof of the blastula contains comparatively smaller blastomeres and the floor is composed of rather large cells.
Gastrulation:
The gastrulation starts with the invagination of the larger cells to form the archenteron. When gastrulation starts, the mitotic activity of the presumptive ecto and mesodermal cells increases but the presumptive endodermal cells remain quiescent. The blastopore is persistent and transforms into the anus.
The gastrula becomes covered with large cilia which help in the rotation of the embryo. Due to the invagination and enlargement of the archenteron the original blastocoel is obliterated and the gastrula becomes double-walled (Fig. 3.21 H). The embryo now elongates along the anteroposterior axis and transforms into a neurula stage.
The neurula stage is characterised by the presence of a neural tube which is formed by the unfolding of the dorsal ectoderm. At this time the innermost layer, i.e., the lining of the archenteron produces two lateral pouches, one on each dorsal side of it.
These pouches are the source of future mesoderm and the cavities are that of the primordial coelom (Fig. 3.21 H). The pouches become subsequently cut-off from the archenteron. The coelom of Branchiostoma is enterocoelous in origin. The notochord is developed from the dorsal-wall of the archenteron.
The overlying layer of the embryo is the embryonic ectoderm which develops into skin and neural tube. The archenteron wall differentiates into the endoderm. The out-pouchings of the archenteron wall transform into the mesoderm.
These pouches proceed ventrally on the two sides of the gut and form the splanchnopleure when applied to the gut-wall and the somatopleure when attached to the body-wall. The splanchnopleure on the sides of neural tube develops into the myotomes and fin-ray boxes.
Larva:
The larva becomes active when it attains two gill-slits and moves about by its epidermal cilia. The mouth develops as a round aperture. The endostyle develops gradually. The larva with eight pairs of gill-slits remains unchanged for a considerable period and many paired gill-slits appear on the pharyngeal wall as a result of subdivision of these gill-slits.
The larva then sinks down to the bottom and becomes metamorphosed into an adult. In a few species of this genus, the larval stage continues for a longer period and the larva even shows the development of gonads. For this reason it was wrongly thought to be a new genus Amphioxus’s.
Remark:
Conclin (1932), Tung et al., (1962), Marshall and Williams (1964), Young (1981), Balinsky (1981),Carlson (1994), Kardong (2002) — all of them have mentioned the larval stage in the life cycle of Branchiostoma (Amphioxus), but according to Anderson (1998) — “There is no larval stage in the life cycle of cephalochordate and the eggs develop into juvenile amphioxus”.
15. Primitive, Degenerate and Specialized Features of Branchiostoma:
Branchiostoma is regarded as an animal in- its organization not far from the vertebrate ancestor. It also possesses a mixture of primitive, degenerate and specialized features. In this respect it resembles many other animals.
Primitive characters:
a. Notochord extends from anterior to posterior except its anterior projection and persists throughout life.
b. Single-layered epidermis.
c. Myotomic segmentation from end to end.
d. The alimentary canal is straight and without loops.
e. The liver diverticulum is simple.
f. Ciliary mode of feeding.
g. Simple circulatory system without a specialized heart.
h. Segmental nephridia which have no coelomoducts.
i. Presence of endostyle.
j. Dorsal and ventral roots of spinal nerves are separate.
k. Gonads are segmentally arranged and without ducts.
l. Absence of biting jaws.
m. Absence of paired fins.
n. Formation of anterior coelomic pouches.
o. Eggs are small and almost yolkless.
p. Blastula is hollow and spherical.
q. Gastrulation embolic.
Degenerate characters:
a. Sedentary in habit.
b. Reduced brain and sense organs.
c. Notochord extending far to the cerebral vesicle.
Specialized characters:
a. Large spacious pharynx.
b. Large number of gill-slits as compared with the body segments.
c. Oral hood, buccal cirri, velum and velar tentacles act as filtering apparatus.
d. Development of atrium reduces and displaces the coelom.
16. Affinities and Systematic Position of Branchiostoma:
Since its discovery, attempts have been made from time to time to determine the biological status of Branchiostoma. A number of groups of animal have been claimed to be related to Branchiostoma.
Many non-chordates have been regarded to be phylogenetically related with Branchiostoma.
Relationship with Annelida:
The concept of annelidan relationship of Branchiostoma was mainly sponsored by Dohrn, Semper and Minot.
Similarities:
(i) The body is bilaterally symmetrical and segmented.
(ii) The nephridia are segmentally arranged and are provided with solenocytes.
(iii) Presence of well-formed coelom.
(iv) Similarity in the disposition of the blood vascular system.
Dissimilarities:
(i) In annelids the segmentation is present throughout the length of the body, whereas in Branchiostoma the segmentation is restricted only to the myotomal region.
(ii) The development of coelom is also different. It is schizocoelic in annelids and enterocoelic in Branchiostoma.
(iii) Although the arrangement of the main longitudinal blood vessels is more or less similar in both, the direction of the flow of blood is diametrically opposite.
(iv) Branchiostoma possesses all the diagnostic features of the chordates, like the notochord, dorsal tubular nerve cord and gill-slits. None of these three structures are observed in annelids, although ‘Faserstrang’ is homologized with the notochord. Faserstrang is a bundle of fibres that supports the nerve cord in annelids.
Relationship with Mollusca:
The ciliary mode of feeding and respiratory mechanism in Branchiostoma resemble closely to that of oysters. At the time of its discovery, Pallas (1778) regarded the specimen to be a slug and named it Limax.
The segmentation in Branchiostoma is a very important characteristic, but in molluscs the body is mostly un-segmented. The molluscan locomotors organ (foot) has no parallel in Branchiostoma. The anatomy of Branchiostoma is completely different from that of molluscs.
Remarks:
The superficial resemblances in the feeding and respiratory mechanisms may be interpreted as the result of physiological convergence for similar mode of living and it has no phylogenetic significance.
Relationship with Echinodermata:
The phylogenetic relationship of the Branchiostoma with the echinoderms is rather important.
Similarities:
The formation of both coelom and mesoderm is strikingly similar in these two groups:
i. The perforations in the calyx of some fossilized carpoid echinoderms are compared with the gill-slits of Branchiostoma.
ii. Biochemical studies also testify the common ancestry of two groups of the animals. The presence of creatine phosphate during energy transfer in ophiuroids and Branchiostoma, the phosphagens in echinoderms and Branchiostoma are the most important supporting biochemical evidences on this line.
Remarks:
For these reasons the echinoderms were formerly thought to hold the key of chordate origin and the structural similarities of Branchiostoma with the echinoderms were held to be due to inheritance from common ancestry. But presently the echinoderms are not regarded as the ancestors of chordates. The similarities are due to a remote common origin of echinoderms and chordates.
Relationship with Vertebrata:
Regarding the relationship between Branchiostoma and the vertebrates two opposing views may be cited:
(i) Branchiostoma might have evolved from some agnathans by degeneration and
(ii) The other view holds that Branchiostoma is the recent derivatives’ of Ascidians. The feeding mechanism and larval similarities speak for a close relationship between Branchiostoma and Ascidians.
Besides these features both of them underwent degeneration and the presence of endostyle establishes biochemical relationship. Branchiostoma possesses many vertebrate features which are lacking in urochordates.
Cephalochordates (Branchiostoma as the typical representative) and vertebrates have a common origin from a stock which is separated from urochordate line. Because of remote phylogenetic connection all these groups are expected to share some common features.
Inclusion of Branchiostoma under the Phylum Chordata is universally accepted, because it possesses the basic features of chordate organisation, viz., the notochord, dorsal tubular nerve cord and gill-slits. But its relative status within the phylum is still uncertain.
Relationship with Hemichordata:
The hemichordates and Branchiostoma show many structural similarities.
The similarities are:
(a) The pharyngeal apparatus has similar structural construction.
(b) Close similarity in feeding and respiratory mechanisms.
(c) The development and arrangement of coelomic sacs are similar. These similarities are due to their emergence from a common ancestor.
In hemichordates the muscles are un-segmented and most of the structures are rudimentary in nature. Besides, the existence of notochord in hemichordates has been questioned and the nervous system as a whole is built more on non-chordate fashion.
Remarks:
Considering all these points, the Hemichordata, if at all considered to be chordates, must be regarded to be primitive than Branchiostoma from the evolutionary point of view and as regards its relative position, Branchiostoma occupies a higher rank than the hemochordates.
Relationship with Urochordata:
The urochordates and Branchiostoma are closely related with one another. The developmental story of urochordates provides the strongest evidence to this view.
The tadpole larva of urochordates resembles Branchiostoma very closely by the following features:
(a) Presence of a continuous notochord.
(b) A hollow nerve cord is located above the notochord.
(c) The pharynx bears endostyle and peripharyngeal grooves.
(d) The tail bears tail-fin.
The adult urochordates are extremely retrograted forms. The feeding and respiratory mechanisms are similar.
Remarks:
Most of the structures are evidently homologous and furnish a convincing evidence of closest phylogenetic relationship between the urochordates and Branchiostoma.
Relationship with Cyclostomata:
Costa (1834) established Branchiostoma to be a jawless vertebrate on the basis of similarities existing between Branchiostoma and cyclostomes. The Ammocoetes larva of lamprey is strikingly similar to Branchiostoma.
Both of them have the following features in common:
(a) The mouth is surrounded by an oral hood.
(b) A velum is present which guards the mouth.
(c) An endostyle is present.
(d) The body bears a continuous dorsal median fin.
Branchiostoma resembles adult cyclostomes, specially by having myotomes, persistent gill-slits and velum. But the absence of cranium, vertebral column and paired sense organs in Branchiostoma goes against the view.
Because of the similarities, many authors regarded Branchiostoma as a permanent larval form of some species of cyclostomes exhibiting a case of ‘paedogenesis’. But such a view requires more supporting evidences in its favour.
Systematic Position:
From the foregoing discussion on the anatomy and affinities of Branchiostoma, the systematic position appears to be uncertain. But its inclusion in the phylum Chordata and subphylum cephalochordate is conclusive. Branchiostoma possesses an admixture of primitive, specialised and degenerated characters.
In spite of the fact, it is regarded to be the vertebrates closest of kin, i.e., it represents a grade of structural organisation not too far from the vertebrate ancestor. Because of its degeneracy and semi sedentary habit, Branchiostoma should not be placed in the direct line of vertebrate evolution. An animal, showing degeneration on one hand and specialisation on the other, cannot possibly react to any evolutionary change.
In his scheme (See Fig. 2.18) Barrington (1965) has summarized the interpretations of many recent workers on this line. It was pointed out that echinoderms and chordates arose from a common sessile or semi-sessile ancestor with external food collection, tripartite body and coelom and ciliated larval stage.
The chordates only developed pharyngotremy. From the ancestral stage where pharyngotremy was established the hemichordates arose in space and time.
The hemichordates retained the external food- collecting habit. On the other hand, internal food collection appeared in some others. This group was the ancestor of all the urochordates, cephalochordates and vertebrates. The urochordates merely arose as a side-branch from this group of animals.
On the contrary, the larva of this group became neotenous and gave rise to free-swimming adults from which the cephalochordates and vertebrates arose. However, leaving aside all such controversies, it can be stated that Branchiostoma represents a fair theoretical picture of a chordate and fulfils the definition of Chordata in its strict sense.