Following steps (sequences) occur in sexual reproduction in a typical angiosperm plant.
A. Development that lead to the formation of male gametes (sperms):
1. Development of anther (microsporangium)
2. Formation of microspore (pollen grain) – micros porogenasis
3. Pollination
4. Development in microspore to form mature male gametophyte and formation of sperms/ male garnets.
B. Development that lead to the formation of female gametes (Egg):
1. Development of ovule (mega sporangium)
2. Formation of megaspore (pollen grain) (mega sporogenesis)
3. Development in megaspore – formation of female gametophyte (embryosac) and female gamete.
C. Fertilization
D. Development of embryo and formation.
A. Developments That Lead To the Formation of Male Gametes (Sperms):
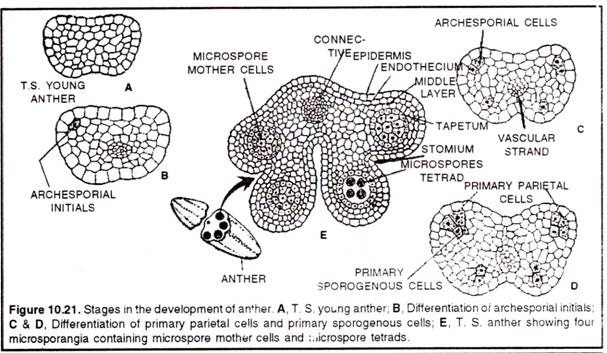
1. Development of anther (microsporangium):
(a) Development of micro-sporangia is eusporangiate type (i.e, from a group of initial cells)
(b) Few cells in the hypodermal region become differentiated as archesporial cells. In Boerhavia and Dionaea, there is only one archesporial cell.
(c) The archesporial cell divides periclinally (along the periphery) to form outer – primary parietal layer and inner – sporogenous layer.
(d) The primary parietal layer lies just beneath the epidermis and divides again periclinally to form 3-5 concentric layers. These layers give raise the wall of the sporangium, along with epidermis.
(e) The innermost layer of the wall is called tapetum, which serves to provide nourishment to the developing pollen grains.
(f) The layer just below the epidermis is called endothecium.
(g) The sporogenous layer may function directly as pollen mother cell or it may divide to form many pollen mother cells.
Anther wall:
(a) The mature anther wall comprises of an epidermis, followed by an endothecium, 2-3 middle layers and innermost tapteum.
(b) Endothecium consists of radially elongated cells, which possess fibrous bands and these are hygroscopic (moisture absorbing) in nature. These help in dehiscence of anther (splitting of anther to release spores).
Tapetum:
It is the innermost nourishing layer of the anther wall present below the middle layer. It is usually single layered and is rich in reserve food material.
It is polyploidy & serves to provide food to the developing sporogenous tissue (microspores) based on its behaviour, tap turn is of two type:
A. Glandular (or secretory) tapetum:
It is also called parietal tapetum. In this type the tapetum cells remains as such in their original position, throughout the microspore development. These cells secrete nutrient materials which are given to the developing spores. It is more common in angiosperms. In the initial stages of .the development, the cells of the glandular tapetum, contains, small bodies, called pro-ubisch bodies, which are involved in the external thickening of the exine of the spore wall.
B. Amoeboid tapetum:
In this type, the breakdown of the inner and radial walls take place and the cytoplasm, containing food material moves into the inner anther cavity and forms the peri-plasmodium mass, which provide nourishment of the sporogenous cells. It is found usually in hydrophytes.
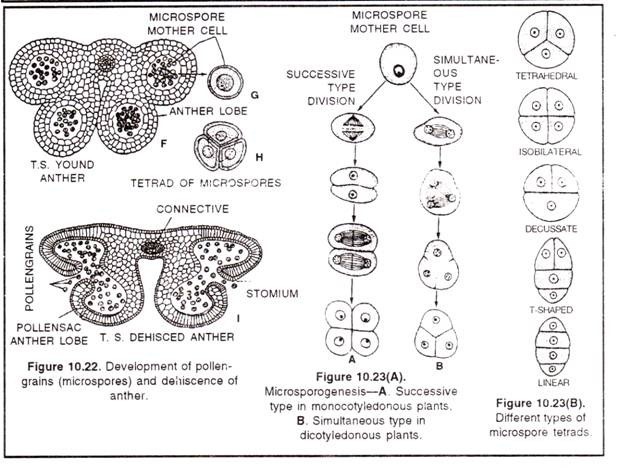
2. Formation of microspores or pollen grains (micro-sporogenesis):
(a) The sporogenous tissues, formed by archesporial cells divide many times to form pollen mother cells (or microspore mother cells) these are diploid cells.
(b) Each such cell divides meiotically (by meiosis) and forms four haploid microspores or pollen grains.
(c) The division is of two types in various angiosperms – simultaneous type and successive type.
(d) In simultaneous division, the M1 of meiosis is not followed by cytokinesis, wall formation takes place only after the completion of both Mi (Meiosis-I) and M2 (Meiosis-ll).
(e) On the other hand, in successive type of division, cytokinesis occurs after both divisions M1 as well as M2
Microspore mother cells →M1 2 haploid cell →M24 Microspore (pollen grain)
The microspore so formed, remain associated with each other for some time. This group of 4n (pollen grains) is called microspore tetrad. Different plants represent 5 types of tetrads -1. Tetrahedral tetrad (most common),
2. Isobilateral tetrad,
3. Decussate tetrad,
4. Inverted T shaped tetrad and
5. Linear tetrad.
In Aristolochiaelegans, all the 5 types of microspore tetrads are found. The enzyme callase disintegrates the callose (polysaccharide) present in pollen tetrad.
Structure of (microspore) pollen grain (microspore):
(a) Microspores (or pollen grains) are the unicellular, uni-cleated, haploid and spherical structures, which develop to give rise to male gametophyte. Smallest pollen are present in myosotis and largest pollens are present in Mirabilis.
(b) Microspores represent the first cell of male gametophyte.
(c) Microspores are surrounded by a two-layered wall.
(d) The outer layer is called exine, which is thick and cuti-cularised layer.
(e) The inner layer is called intine, which is smooth, thin and cellulosic.
(f) The exine shows different types of outgrowths. It contains a resistant fatty substance, called sporopollenin. Exine consists of extexine (outer) and endexine (inner) Extectine further coisists of a foot layer, baculate layer(middle) and an outermost tectum. Tectum characteristic texture to exine. The morphology and texture of exine is important from taxonomic point of view. The study of pollen and its exine structure is called palynology. Father of palynology is Erdtman & Indian palynology is P.K. Nair.
(g) Chemically the pollen grains are composed with carbohydrates (25-48%), protein (7-26%), and water (7-16%), Fats (1-15%).
(h) The germ pores are important because these mark the origin of pollen tube,
(f) Normally there are three germ pores in dicots, while only one in monocots.
Pollinium (Translator Apparatus) Corpusulum:
In some plants of family Asclepidiaceae, (as in Calotropis procera) orchidaceae (orchids) the spores remain together in a single mass, called pollinium. Pollinium occurs in pair forming balloon like structures. These structures are called pollinia. Each pollinium consists of a stalk (called corpusculum), caudicle (disc like) and two pollinia carries mass of pollen grains.
Germ pores:
(a) These are the weak portions of the exine of the pollen grain. These are the points where from germ tube (pollen tube) emerges out, during germination.
(b) Some pollen has one germ pore, while others have two or three germ pores. If only one germ pore is present the pollen is called monocolpate.
(c) If two pores are present the pollen is called bicolpate and three germ pores are called tricolpate.
3. Development in Microspore and Formation of Male Gametophyte:
Development of male gametophyte starts when the pollens are within the anther lobes. Thus initial development of male gametophyte takes place inside the anther lobes (micros-porangia). But when half of the germination (i.e, up to 2-3 cell stage) gets completed, the anther dehisces and the germinating pollens (male gametophyte) are shed. Now pollination takes place (i.e, these pollens are transferred to the stigma of the carpel of a flower). Further development of male gametophyte take place on the stigma of carpel.
The development of the male gametophyte involves following steps –
(a) Development before pollination.
(b) Dehiscence of anther and liberation of developing pollens.
(c) Pollination and
(d) Development after pollination
(a) Development of male gametophyte before pollination (Micro-gametogenesis)
1. Development of male gametophyte before pollination occurs inside the anther (microsporan gia)
2. During germination, the nucleus of the microspore is displaced from centre to one side. This displacement is always in a particular direction and marks the position of the germi-native cell.
3. The cytoplasm becomes highly vacuolated.
4. Microspore undergoes mitotic division and forms two unequal cells:
a. The larger cell, called vegetative cell and
b. The smaller cell, called generative cell or germinative cell.
5. The vegetative cell on germination gives rise to pollen tube (after pollination, on stigma)
6. The germinative cell produces two sperms (male gametes) by one more mitosis, later.
7. The germinative cell is initially attached to the wall of pollen grain, but later comes to lie freely in to the cytoplasm of vegetative cell.
8. Generally the pollens are shed (fall) at two-cell (vegetative and germinative cell stage), and further development of the male gametophyte takes place on the stigma, after pollination. In some cases, the pollen is shed at three celled stage (vegetative cell and two sperms stage). Thus, in these plants, the sperm formation takes place before pollination.
(b) Dehiscence of anther and liberation of developing pollens. When pollens get matured (usually at 2 celled stage), they exert some pressure on anther wall. Consequently the wall of the anther gets burst and sets free the germinating pollen grains. This is called dehiscence of anther.
Thus, the gametcphyte is reduced to three nuclei, only one of which is not a gamete.
C. Pollination:
Transfer of pollen grains from anther to the stigma (of a pistil) is called pollination.
It is of two main types:
(i) self-pollination and
(ii) Cross-pollination.
(A) Self pollination:
Transfer of pollen from anther of a flower to the stigma of the same flower (or flower of same plant) is called self pollination. Self-pollination can be two types, autogamy and geitonogamy,
(1) Autogamy:
It is a type of self-pollination that is found only in bisexual flower. In this case, the stigma of a flower is pollinated by its own pollen. Autogamy occurs by three methods.
(a) Homogamy:
The anthers and stigmas of open flowers are brought together by growth, bending or folding. Ex-Catharanthus (Vinca) Mirabills (four O’clock plant)
(b) Cleistogamy:
The (lowers remain closed during pollination (with the help of its petals) Thus, stigma receives only the pollen of its own flower. Ex- Oxalis,Arachis hypogea, Commelina, Viola etc. 11 is best contrivance for self pollination.
(c) Bud Pollination:
In this case the pollination occurs in the bud stage. The sex organs develop before the opening of bud, thus internal pollination takes place. Ex- Pea, Wheat, Rice etc.
(2) Geitonogamy:
In this type of pollination, the pollen grains of one flower are transferred to the stigma of another flower in same plant or genetically similar plant.
Advantages of pollination:
(a) It maintains purity of the species, by preserving all the parental characters
(b) It is used to obtain pure-line characters (homozygosity) during breeding experiments.
(c) Plant does not have to depend on pollinating agencies.
(d) Only a small number of pollen grains are required
(e) Self-pollination strengthens the better characters of the plant.
Disadvantages of self pollination:
(a) It does not eliminate bad characters from the race.
(b) Vigour and vitality of the race decreases, as there is no hybrid vigour.
(c) Immunity to diseases decreases.
(d) Ability to adapt according to changing environment decreases.
(e) No role in evolution.
Cross pollination/Allogamy:
Transfer of pollen grains from the anther of the flower of one plant to the stigma of the flower of other plant by the help of agents is known as cross pollination. Agent may be biotic (non living) or Biotic (living).It is commonly seen in dioecious plant but very rarely in monoeclous. It is highly advantageous than self pollination mainly in formation of new genotypes.
Types of Cross Pollination:
Cross pollination by abiotic (non Lining) agents:
1. Pollination by Wind or air is known as Anemophilly.
2. Pollination by Water is known as Hydrophilly.
Cross pollination by biotic (living) agents.
1. Pollination by insect is known as Entomophilly.
Ex – Salvia, Cestrum, Ficus
2. Pollination by bird is known as Ornithophilly. Ex – Bombax, Callistemon
3. Pollination by animal is known as Zoophilly.
4. Pollination by Snail is known as Malacophilly. Ex. Lemna, Colocasia, Diptera
5. Pollination by Bat is known as Cheireptrophilly.
Ex. – Kigelia, Anthocephalus, Adansonia, Bauhinia
6. Pollination by ant is known as Mirmicophilly.
7. Pollination by Snake is known as Ophiophilly.
Contrivances (adaptations) for Cross- Pollination:
The cross-pollinated plants are seen to adopt several devices for the success of cross-pollination. Some of-these adaptations are;
1. Uni-sexuality or Dicliny:
Often uni-sexuality is of great help in the success of cross-pollination. Plants bearing unisexual flowers are dioecious (e.g. Cannabis, papaya). It is best contrivance for cross pollination.
2. Self-sterility or incompatibility:
Some plants, such as Passiflora, Potato, Malva Abutilon show self-incompatibility, because in these plants pollen grains from an individual flower cannot fertilize its own ovules as these pollen-grains fail to germinate on stigma of the same flower. Self- sterility in plants is under genetic control which prevents the ovules being fertilized by pollen from the same plant.
3. Dichogamy:
It refers to the device when in some hermaphrodite flowers stamens and carpels of a flower do not mature at the same time.
Dichogamy is of two types:
(i) Protandry:
When anthers mature much earlier than the carpels of a flower, e.g., Sunflower, Tagetes, Jasminum, Foeniculum etc.
(ii) Protogyny:
When carpels of flower mature much earlier than its anthers, e.g. Rose, Ficus benghalensis, Polyalthea (Ashok) etc.
4. Heterostyly:
Sometimes due to great disparity in the length of style and stigma, effective self pollination is not possible, e.g., Primula, Lathynis, Oxalis etc.
5. Herkogamy :
In some cases, the homogamous flowers adapt some unusual devices for successful cross-pollination. For instance, in caryophyllaceous flowers, the stigma grows much beyond the limits of stamens so that its own pollen-grains fail to read its own stigma. In Calotropis, the corolla act as hood in between and androecium and gynoecium.
Advantages of Cross Pollination:
1. It helps to eliminate bad characters from the race.
2. It helps in the development of new characters due to recombination of genes.
3. Vigour and titality of the race increases, due to heterosis.
4. Immunity to diseases increases.
5. Ability to adapt according to changing environment increases.
6. Produces new genotype & has important role in evolution.
Disadvantages of Cross-Pollination:
1. It is a highly wasteful method as plants have to produce large number of pollen-grains since much of it may not reach the stigma of the right flower and get lost during transit.
2. Cross-pollination is not a sure method as chance factor is always there.
3. It is less economical as the plants have to develop many devices to bring about this kind of pollination through various external agencies.
4. Some of the very good traits of the race are likely to be lost during recombination.
5. There are chances of the en try of some harmful and undesirable traits in the plants, and the same may persist in the race for ever.
Various agencies of cross pollination (Allogamy):
Most of the plants are chasmogamous type (i.e, they expose their anthers and stigma to the pollinating agencies). As the pollen grains do not have locomotory structures, they are transferred from anther to the stigma, with the help of certain agencies, called as pollinating agencies. Some of them are wind (air), water, insects, bats, birds and even by man.
1. Anemophily:
Anemophily refers to the pollination by wind (air). Wind pollinated flowers (a primitive features) show following characters:
(a) Flowers are inconspicuous and not showy.
(b) They are devoid of fragrance and rectar.
(c) They produce a very large amount of pollen, grains, as considerate amount of pollen never reaches the proper stigma.
(d) Anthers are versatile, swimming freely in air.
(e) Pollen grains are dry, light and smooth walled.
(f) Stigma is large, blanched and bushy capable of catching pollen from air easily, as in cereals.
2. Hydrophily:
Pollination through water current is called hydrophily. Aquatic nature of the plant is no identification of its being pollinated through water. Many hydrophytes are pollinated through wind (Patamogeton) or insects (Alisma, Nymphaea). when it is with the help of water, it may take place completely under water (hypohydrophily) or may takes place on the water surface (epihydrophily).
Hydrophilous plants, like anemophilous flowers are characterised by floral envelops which are highly reduced or even absent. It is commonly found in plants like Zostera(Manne angiosperm), Ceratophyllum and Vallisneria.
3. Entomophily:
Insects are the chief pollinators and it has-been noted that the evolution of flowering plant has gone hand-in-hand with the evolution of the insects. Insects helping in pollination are bees, flies, beetles and moths. Of these the first three are diurnal visiting flowers which opens in day time and moths are nocturnal i.e. they visit flowers which open after sunset.
Insects pollinated flowers show following characters:
(a) Flowers are brightly coloured.
(b) Pollen grains, stigma are sticky with a rough surface, so that they may easily stick, to insect limbs.
(c) A sugary fluid called nectar or honey is secreted in many plants. Insects visit flowers for nectar. Nectar glands may be situated on thalamus, sepals, petals, carpels or base of ovary.
(d) In some entomophilous flowers, special mechanism are seen. Night bloosming flowers have white colour and fragrance (so that they may be visible to insects).
(e) In Centaurea (compositae), pistil bends and exposes the stigma on being touched by insects. In Salvia, versatile anthers and other balancing features, help in dusting of insects with pollen.
4. Ornithophily:
Birds pollination is not so common. Humming – birds, sun birds and honey eaters are some of the birds which visit flowers and bring about pollination. Such flowers are tubular, cup-shaped or urn shaped, bright in colour and produce large quantities of pollen and plenty of nectar.
5. Cheiropterophily:
In this case the pollination is carried out by bats. Chiropterophilous plants have flowers borne singly or in clusters quite away from branches and leaves due to their long stalk.
These flowers open only at or after dusk. On blooming, flowers emit an odour and produce large quantities of nectar. Flowers are dull in colour. Bats, being nocturnal are attracted by the odour of the flowers.
6. Malacophily:
Snails and slugs visit certain flowers and may be playing a role their pollination. This is generally observed in plants like Arisaema (cobra plant) and also in arum lilies.
Development of male gametophyte after pollination:
It involves following steps:
(i) Formation of male gametes (sperms):
(a) Only a right kind of spore germinates on the right kind of stigma, (i.e.; for germination, both pollen and the stigma must be of the plants, that belong to the same species or genus.)
(b) After pollination, the male gametophyte (germinating pollen) is usually at two cell stage. It contains a vegetative cell and a germinative cell or generative cell.
(c) The germinative cell contains a large amount of protoplasm. It acquires a characteristic vermi form appearance. It now divides mitotically, to form two elongated, haploid male gametes or sperms.
(ii) Formation and growth of pollen tube:
(a) The cytoplasm of the vegetative cell bulges out through germ pore, in the form of a tube, called pollen tube.
(b) Pollen tube at the apex, contains tube nucleus (which is in fact the nucleus of vegetative cell)
(c) Behind the tube nucleus, there are 2 male gametes (sperms)
(d) The tube grows towards the ovule (megasporangium), making its way through the style.
(e) Usually, a single pollen tube arises from one pollen. Such pollen grains are called monosiphonous. However in some plants, pollen grains give rise to many pollen tubes. Such pollens are called polysiphonous.
Now pollen tube makes its way through the style (of carpel) and move towards the mature ovule containing female gametophyte (embryo sac) absorbing chemicals borate & calcium from style. Movement is chemotropism.
1. Development of Ovule (Megasporangium):
(a) Ovule arises as a small mound of homogenous tissue on the inner wall of the ovary (placenta).
(b) This mound develops to form the inner central part of the ovule, called nucellus.
(c) Integumentary’ processes arise close to the base of this mound, which forms outer covering of the ovule.
Structure of ovule (mega sporangium):
(a) Each ovule is attached to the inner wall of the ovary (placenta), by a slender stalk, called funicle.
(b) The point of attachment of ovule to its funicle is called hilum.
(c) Main body of the ovule is formed by inner central mass i.e., nucellus. Nucellus consists of living parenchymatous cells.
(d) Each mature ovules the nucellus serves to cover and provide nutrition to the embryo sac (female gametophyte).
(e) Each ovule has two distinct ends-a micropyle end (it also called opening of ovule during fertilisation) and b. Chalaza end (the posterior end, opposite to micropylarend).
(f) Externally the nucellus is covered by one or two protective covers, called integuments. These integuments arise from the chalazal end.
(g) When only one integument is present, the ovule is called unitegmic, and if the ovule consists of two integuments, it is called bitegmic very rarely tri-tegmic (with three integuments) is present in plants like Asphodelus. In some plants such as Santalum, etc, ategmic (no integument) condition may be present.
(h) In mature ovules, the female gametophyte or embryo sac is present in the centre. The embryosac consists of egg cell (female gamete), synergid cells, antipodal cells and polar nuclei, (this is described a little later).
Types of Ovules:
On the basis of position of micropyle, with respect to the funiculus, ovules are 6 types:
1. Orthotropous ovule:
It is atropous or straight, where the micropyle, chalaza and the funiculus, all are in the same line. Ex- Cycas, Family Polygonaceae and Piperaceae.
2. Anatropous ovule:
It is of the most common occurrence more than 80% of angiosperm family). In this ovule, the funicle is long whole body of the ovule is inverted, through 180°. As a result the micropyle comes close to the funicle. Ex-Most common in dicots and monocots, Ex Asteraceae, Solanaceae.
3. Hemianatropous or hemitropous ovule:
In this case the body of the ovule is inverted only through 90°. As a result the funicle comes to lie at right angle to the nucellus. Micropyle and chalaza, lie in the same plane Ex-Ranunculus.
4. Campylotropous ovule:
When body of the ovule is not completely inverted, but is it bent like-‘horse shoe’. The micropyle and chalaza do not lie in the same plane (however the nucellus/ embryo-sac remain straight). Ex-Family Capparidaceae, Cruciferae (Brassicaceae), Carypohyllaceae, Fabaceae etc.
5. Amphitropus ovule:
It is similar to campylotropous, but in the case the nucellus/embryo-sac is also bent like ‘horse shoe’ Ex- Family Alismaceae,
6. Circinotropous ovule:
It is of a very rare occurrence. Here the body of the ovuyle is bent through 360°, so that it takes a one complete turn. (Micropyle, chalaza and the nucellus are all in same plane). Ex-Opuntia
2. Formation of Megaspore (Megasporogenesis):
(a) In the micropylar region of the nucellus, usually a single hypodermal cell gets differentiated from other cells. It is called primary archesporial cell. It contains a big nucleus, dense cytoplasm and has a larger size.
(b) This cell divides periclinally, to form primary parietal cell and primary sporogenous cell.
(c) The parietal cell may either remain undivided or undergoes a few periclinal and anticlinal divisions, so that the sporogenous cell gets embedded in the nucellar mass.
(d) Consequently the sporogenous cell becomes sub-hypodermal in position. Ovules with such ahypodermal sporogenous cell are called crassinucellate
(e) The sporogenouscell now acts as megaspore mother cell. It undergoes meiosis and forms fourhaploid megaspores.
(f) In most of the angiosperms, out of these 4 megaspores, 3 get degenerate (to provide more nourishment to the remaining one). Functional megaspore commonly present towards chalazal end .Thus only one megaspore remains in the ovule. This gives rise to female gametophyte on development, (such female gametophyte or embryosac which develops from single megaspore, is called monosporic embryosac or polygonum type. In some angiosperms bisporic or even tetrasporic embryosac may also be present).
(g) A megaspore is a haploid structure and represents the first cell of the female gametophyte. It develops to form fully matured gametophyte.
3. Formation of Female Gametophyte (Embryosac):
Female gametophyte is also called embryo sac. It develops from the functional megaspore. There are great variations in the development of embryo sac. Some are monosporic, some others are bisporic and rest others are tetrasporic, as discussed above. (We are describing here the development of a typical embryo sac, which is monosporic and is of the most common occurrence among angiosperms).
(a) The remaining megaspore in the ovule is given sufficient nourishment, so that it becomes larger in size.
(b) The megaspore now divides by three successive mitotic divisions and forms 8 nuclei. All theses nuclei are haploid.
Haploid megaspore →2 nuclei → 4 nuclei → 8 nuclei
(c) Of these 8 nuclei, 3 nuclei (at micropylar end), undergo cytokinesis (forming cell membrane) and form egg apparatus.
(d) The egg apparatus contains 2 synergid cells and one egg cell.
(e) The egg cell represents the female gamete.
(f) Other three nuclei (at chalazal end) also undergo cytokines is and form three antipodal cells.
(g) Remaining two nuclei ire present in the centre (they do not follow cytokinesis). These are called central or polar nuclei or definitive nuclei.
(h) This entire structure is called embryo-sac, which represented the mature female gametophyte. Thus normally it contains 3+2+3 arrangement of cells in a typical embryo sac.
(i) The mature female gametophyte (embryo-sac) consists of 8 nuclei, but only 7 cells (one egg cell, 2 synerdids, 3 antipodal cells and one remaining cell, in which 2 polar nuclei or one secondary nucleus are present).
Structure of mature embryo sac (Female gametophyte):
The mature female gametophyte or embryo sac in a typical angiosperm is 7 celled and 8 nucleated. It contains one egg cell, 2 synergids, 3 antipodal cells and 1 largest central cell with 2 polar nuclei.
Synergids:
These are 2 elongated ceils, present at the micropylar end of the ovule, one on each side of the ovule. The characteristic feature of the synergid cells is the presence of finger like filliform apparatus. It is developed due to chemotactic stimulus; it transports the secretion products towards the micropylar tip of egg apparatus where pollen tube establishes contact with the embryo sac.
Synergids play an important role in directing the pollen tube growth, by secreting some chemotropically active substances like sucrose. The degenerating synergids help pollen tube to discharge and release its contents, in the embryo- sac. Some workers have suggested its haustorial or nutritive function.
Antipodals:
(a) These are usually 3 in number, present at the chalazal end of the embryo-sac.
(b) These exhibit the great variation in size, structure, life span and biochemistry.
(c) Usually these cell degenerate before/soon after fertilisation, (but in Caltha pulustris the an tipodals persist upto the stage of the pro-embryo).
(d) Antipodals cells serve to provide nutrition to the pro- embryo.
Egg cell:
(a) It is a single haploid cell, at the micropylar end, between two synergids.
(b) It represents the female gamete.
(c) It when fertilised by sperm (male gamete) forms diploid zygote. 
3. Fertilisation:
Fertilisation is angiosperms, is unique. It is called double fertilisation. This is because there are two sperms per pollen tube. One of the sperms fertilises the egg cell to form zygote, while remaining sperm fertilises two polar nuclei, resulting in the formation of a triploid endosperm cell. The phenomenon of fertilization was first reported by Strasburger (1884) in Monotrapa. The male gametes are brought to the egg present in female gametophyte by a pollen tube. This phenomenon is called siphonogamy which was discovered by G. B. Amici in Protulaca plant.
Important events in fertilization are following:
1. Entry of pollen tube into ovule and embryo sac:
(a) After arriving in the ovary, the pollen tube finds its way through style and enters the ovule.
(b) Depending on the place, the retry of pollen tube into the ovule, can be of following three type:
(i) Porogamy:
It is the most common type. Here the pollen tube enters the ovule from the micropylar end. According to some workers, the porogamy is directed by filiform apparatus (of synergid).
(ii) Chalazogamy (Basigamy):
In this case the pollen tube enters the ovule from chalazal end; however the entry into the embryo sac occurs through micropyle. Ex- Casuarina.
(iii) Mesogamy:
In this case the pollen tube enters the ovule through the funiculus or integuments. However the final entry into the embryo sac occurs through micropyle. But the pollen tube with two mole gametes enters into the embryosac only through the micropyle.
Obturator:
It is a special structure which facilitates the entry of pollen tube into the ovule, by forming a sort of bridge. Soon alter fertilization the obturator shrinks and disappears.
2. Discharge of male gametes (sperms):
(a) The pollen tube contains two sperms (each is a haploid male gamete).
(b) When pollen tube enters the embryo-sac (inside the ovule), it bursts to release its contents i.e., two sperms along with certain amount of protoplasm .The first male gamete discharge in one of the synergid.
3. Double fertilization:
(a) Both the male garnet present in pollen tube utilises in fertilisation process of angiosperm is known as double fertilisation. It is the characteristic feature of angiosperms except Family Orchidaceae, Podostemaceae and Trapaceae.
It involves two types of fusion –
a. Syngamy (fusion of egg cell and one male gamete) and
b. Triple fusion (fusion of remaining male garnet and two proper nuclei).
c .It was first observed by Nawaschin (1898) in Fritilaria and Lilium. It was supported by Guignard (1899).
(1) Syngamy:
(a) One of the two sperms goes to fertilize the egg cell. This fusion is called syngamy.
(b) It results in the formation of zygote, which gives rise to proper embryo.
(2) Triple fusion:
(a) The remaining sperm now fuses with the two haploid polar nuclei (present in the centre of embryo sac).
(b) This fusion is called as triple fusion (as three nuclei i.e., one male garnet and 2 polar nuclei, are fused).
(c) It results in the formation of triploid endosperm nucleus, which on development (Repeatd mitosis) form the endosperm.
(d) Endosperm is therefore triploid in angiosperms (It is a characteristic feature of angiosperms)
(e) Endosperm serves to provide nutrition to the developing embryo.
Development of Embryo (Embryogeny):
As written earlier also, the highly organized body of a seed plant represents the sporophytic phase of the life-cycle. It begins its existence usually with the fertilized egg, the zygote, which develops into the embryo by characteristic steps showing characteristics of future adult organization of the plant. Embryo, as a whole, assumes a specific form in which an axis and one or more leaf like appendages, the cotyledons, can be recognised.
Because of its location below the cotyledons, the stem like axis is called hypocotyl. At its lower end (i.e., the root pole), the hypoectyl bears the incipient root, at its upper end (i.e., the shoot pole), above the caryledons, the incepient shoot. The root may be represented by its meristem (apical meristem of the root) or by a primordial root, the radicle. Similarly, the apical meristem of the shoot located at the shoot pole may or may not initiate the development of a shoot above the cotyledons. If a primordial shoot is present, it is called epicotyl or plumule.
(i) Development of Embryo in Monocot Plants:
In monocotyledonous plants, a good deal of variation is found in the stages of development of embryo. It is rather difficult to find a single plant in which the development of embryo may be considered as typical of monocotyledons. Development of embryo in Sagittaria (family, Allismaceae) has close resemblance to that in other monocots and is thus described here.
Soon after fertilization, the fertilized egg or oospore greatly enlarges in size and divides transversely to form a 3-celled proembryo. These three cells are basal, middle and terminal. The basal cell, which is cut off towards the micropylar end, enlarges very much and forms major portion of the suspensor. The middle cell undergoes repeated transverse and vertical divisions thus differentiating few suspensor cells, radicle, plumule and hypocotyl. The terminal cell also undergoes a number of divisions in various planes and forms a single cotyledon.
Here the cotyledon is a terminal structure and the plumule is laterally situated in a depression. In some monocots, like Colocasia, there is no suspensor in the embryo. Suspensor usually pushes the embryo into the endosperm from where the former gets nourishment. The suspensor may also serve as haustorium to absorb food from the nucelius. In some monocotyledonous plants like Agapanthus (family, Liliaceae) two cotyledons are formed instead of one.
(Ii) Development of Embryo in Dicot Plants:
Development of embryo in dicotyledonous plant basically follows a uniform pattern except for slight variations. The fertilized egg or oospore greatly enlarges in size, elongates and divides transversely into a suspensor cell (towards the micropylar end) and an embryonal cell (towards the middle of the embryo sac). As the development proceeds, the suspensor cell further divides and forms a 8 to 10-celled suspensor which pushes the developing embryo into the food storage tissue, endosperm. The lowermost cell of the suspensor, which is called hypophysis, undergoes more divisions to form the radicle.
These changes in the suspensor cell are associated with the enlargement of the embrynonal cell. The embryonal cell also divides; the first division is longitudinal followed by one more longitudinal at right angle to the first and the other transverse forming an octant (8-celled proembryo). This is followed by a periclinal division thus forming a 16-celled structure which may be differentiated into posterior or hypobasal octant (towards the suspensor) and anterior or epibasal octant. The epibasail cells, as a result of further divisions and differentiation, form plumule and cotyledons, while the hypocotyl is formed from the hypobasal cells.
The peripheral cells of the proembryo subsequently divide by anticlinal walls, thus differentiating a single-layered dermatogen (it forms epidermis). The middle-cells of the proembryo also divide periclinally and form plerome (which forms vascular tissue). The tissue in between dermatogen and plerome is known as periblem (it forms the cortex).
As the development further proceeds, the free end of the developing embryo becomes heart-shaped, The two lobes of the ‘heart’ are the primordia of the cotyledons. Plumule is situated in the depression in between two cotyledons. Unlike monocot embryo, here the cotyledons are lateral and the plumule is terminal.
As in Capsella bursa pastoris, the embryo enlarges rapidly consuming the surrounding endosperm. Thus the seed so formed becomes non-endospermic. The endosperm may not be completely exhausted and persists in the seed; such condition is found in endospermic seeds leg:, castor).
Development of Endosperm:
The endosperm, a food tissue of varying degree of importance in different species of angiosperm plants, is formed in most cases as a result of fusion of the two polar nuclei and one male gamete. Since all three of die fusing nuclei are haploid, the endosperm is triploid. In the families Orchidaceae and Podostemonaceae, the endosperm formation is completely or partly suppressed.
Types of endosperm formation:
Three types of endosperm formation has been reported in the angiosperms:
(1) Free nuclear type.
(2) Cellular type.
(3) Helobial type.
(1) Free nuclear type of endosperm:
The first and usually several of the following divisions of the primary endosperm nucleus are not accompanied by cell wall formation, The endosperm nuclei may either remain free or, in later stages, they may gel separated by cell-walls. The divisions of the endosperm nuclei are quite irregular and, in an endosperm, can be seen in different stages of divisions. As divisions progress, the nuclei are pushed more and more towards periphery that the centre is occupied by a large vacuole.
Quite frequently, the endosperm nuclei in the chalazal part of the embryo sac have been observed to be larger than those in the micropylar region. The number of free nuclear divisions varies in different species of plants, for example in Primula, Mangifera, Malva, Mains Cannabis etc. several hundred endosperm nuclei may be seen lining the wall of the embryo sac.
In Asclepias, Rafflesia and Calatropis etc., the cell wall formation starts at the very early stage when only 8 to 16 nuclei have been formed, and in Coffea cell wall formation occurs at the 4-nucleate stage. The cell wall formation starts from the periphery of the embryo sac. Formation of endosperm haustoria has also been reported in several plants e.g., members of family Proteaceae (Kaushik, 1938, 1942).
(2) Cellular type of endosperm:
Here the first division of the primary endosperm nucleus results in the partition of embryo sac into two chambers. The first wall is usually transverse (e.g., Villarsia raniformis, Impatiens roylei, Ruellia etc.,) but sometimes vertical (e.g., Adoxa, Scabiosa, and Circaeastere etc.,) or oblique (e.g., Paperomia, Centranthus, and Helosise to.,), and in few cases the plane of division is variable (e.g., Senecio). Endosperm haustoria may also develop at the micropylar or chalazal ends. In the family Scrophulariaceae, both micropylar and chalazal haustoria are formed.
(3) Helobial type of endosperm:
It is an intermediate type between the nuclear and the cellular. Here the first division of the primary endosperm nucleus results in the chambering of the embryosac. The micropylar part of the sac is usually larger than the chalazal part. The endosperm nucleus in the micropylar chamber undergoes several free nuclear divisions, while nucleus in the chalazal region either remains undivided or undergoes only a few divisions, (e.g., Eremurus)
Structure of the endosperm:
The endosperm, as stated earlier, is a food tissue of varying degree of importance in different species of angiosperms. Its cells are mostly isodiametric in shape and store large amount of food materials. Although the cell-walls are usually thin and devoid of pits. In majority of the grasses and some other plants, an aleurone layer is present on the outside of the endosperm. Possibly, the aleurone layer secretes certain enzymes which transform the stored food into liquid form so that it may be consumed by the developing embryo.
In fully ripe seeds, the endosperm represents a physiologically dead tissue because of the degeneration of the nuclei of the endosperm cells. Dying of the nuclei in the endosperm cells, however, promotes the filling of the grain, and embryo can secure the food material more easily from a dead rather than a living tissue.
Endospermic and Non-Endospermic Seeds (The Final Fate of Endosperm):
1. Endospermic Seeds:
In some seeds the endosperm forms a permanent tissue (e.g., Ricinus, Phoenix. Triticum) which persists, enlarges and become a prominent tissue of the seed, rich in accumulated food in the form of oil, starch and proteins. The food stored in the endosperm is utilized by the embryo when the seed germinates. Such seeds are called endospermic or albumionous seeds.
2. Non-endospermic Seeds:
In other seeds (e.g., Cucurbits, Pisutn, Arachis) it is used up by the growing embryo and is no longer seen in the mature seed. Such seeds are called non-endospermic or ex-albuminous seeds. Of special interest is Symplocarpus in which the embryo consumes not only the endosperm but also integuments of the ovule so that the embryo lies naked inside the ovary wall. An even more extreme case is that of Melocanna bambusiodes in which embryo dissolves even the ovary wall and lies completely naked at maturity.
Xenia; Metaxenia, Mosaic Endosperm and Ruminate Endosperm:
1. Xenia:
In plants like maize the influence of male garnets is seen on the development of endosperm. If the male parent has a yellow endosperm and female parent a colourless endosperm, after fertilization the endosperm of the newly formed seed shows yellow colour. This transference of characters by a male gamete and its influence on endosperm is known as Xenia (Focke, 1881).
(2) Metaxenia:
The effect of pollen on the character of the seed coat or pericarp is called metaxenia and this term was given by single in 1928. It was observed in Phoenix dactilifena.
(3) Mosaic endosperm:
Occasionally there is a lack of uniformity in the tissue of endosperm. The patches of two different colours are often observed in Zea mays forming a sort of irregular pattern. Some part of the endosperm may be starchy while the other part may be sugary. It was observed by Webber in 1900.
(4) Ruminate Endosperm:
Mature endosperm showing irregularity or unevenness in its surface contour is called ruminate endosperm (e.g.. Passiflora calarata, Cocoloba). Accoridng to Pariasamy (1963), ruminations arise due to in growth or infolding of the seed coat or by unequal radial elongations of the seed coat cells.
Morphological Nature of Enosperm:
Morphological nature of endosperm of flowering plants is very much disputed one. In gymnosperms, endosperm is a gametophytic tissue (In) because it develops from megaspore nucleus before fertilization and thus haploid (In) in nature. But, in angiosperms endosperm develops by the fusion of 2 polar nuclei and 1 male gamete, hence, it is a triploid (3n) structure. Different scientists have different opinion about morphological nature of the endosperm of angiosperms.
1. According to Monnier (1890) and Ms. Sargent, it is a sporophyte.
2. According to Strasburger (1900), Coulter and Chamberlain (1911), endosperm is a gametophyte.
3. According to Brink and Cooper (1940), it is an entirely new structure.
According to them, double fertilization is a device to compensate for the extreme reduction of female gametophyte in angiosperms. Due to extra chromosome it is more effective in obtaining food for the embryo from the nucellus and the integument.
Parthenocarpy:
Development of fruits without fertilization is called parthenocarpy and the fruits thus produced are known as parthenocarpic fruits or seedless fruits, e.g. Musa (Banana), Psidium (Guava), Pyrus (Apple), Vitis (Grapes) etc. The term parthenocarpy was coined by Noll (1902).
Types of Parthenocarpy:
Nitsch (1963) has recognized three types of parthenocarpy:
(a) Genetic parthenocarpy:
Several cultivating plants have both seeded as well as seedless fruits. These seedless fruits are formed parthenocarpically due to hybridization or mutation. The famous seedless navel variety of orange was developed from a normal seed-bearing variety of Citrus through mutation in axillary bud that grew out into a branch bearing seedless fruits.
(b) Environmental parthenocarpy:
Environmental conditions such as fog, frost, high temperature, freezing interfere with the normal functioning of reproductive organs and bring about parthenocarpy in plants e.g., Heavy fog during month of June helps formation of seedless fruits in olives (Campbel, 1935), parthenocarpic fruits in Capsicum by keeping the plant at relatively low temperature (6° to 10°C) at the time of an thesis (Cochran, 1936), in pears by placing its flowers to freezing temperature for 3 to 19 hours (Lewis, 1942).
(c) Chemically induced parthenocarpy:
Thimann (1934) gave the idea that pollen grains have auxin and other growth regulatory substances that have stimulatory effects on the female sex organs. Artificial application of 0.5 to 1.0%. Solution of 1AA (Indole Acetic Acid), α-Naphthalene acetic acid) para chlorophenoxy acetic acid, phenyl acetic acid, gibberellins etc.
Significance of Parthenocarpy:
1. Parthenocarpic fruits have an increased proportion of edible part than in normal fruits.
2. In horticulture seedless fruits are suitable either as consumption or in the preparation of jams and juices.
Polyembryony:
Presence of more than one embryo inside the seed is known as polyembryony. It was first reported by A.V. Leuwenhoek (1719) in Citrusi Fam-Rutaceae).This phenomenon is very common in gymnosperm than angiosperm. Besides normal embryoe (develops from Zygote), other embryos are formed inside seed maybe as haploid (n) or Diploid (2n).
Types of polyembryony:
Polyembryony can be broadly categorized in to two groups.
1. True polyembryony: Many embryos are developed inside single embryo sac.
2. False polyembryony: If the ovule carries more then one embryo sac & embryos develop in each embryo sac.
Cause of polyembryony in angiosperm:
Polyembryony takes place due to:
1. Cleavage of proembryo:
It is simplest method of polyembryony where zygote divides into many units & each unit develops an embryo.
2. Development of many embryo from synergid, antipodal cells, endosperm except egg.
3. Development of many embryo due to presence of more than one embryo sac.
4. Development of polyembryo from nucellus, integument (outside the embryo sac) According to Haberiandt (1921)” Stimulus for polyembryony is provided by degenerating cells nucelleus.(Necrohormone theory)
Apomixis:
Sexual reproduction (Amphimixis) normally carries two regular features i.e Meiosis & Fertilisation. But in some pants abnormal kind of amphimixis takes place in which egg or cell in embryo sac (synergid, antipodal cell) develop into an embryo without fertilisation and with or without meiosis.
“Apomlxis is abnormal type of sexual reproduction where there is no meiosis & fertilisation.”
Types of Apomixis:
It is mainly of two types:
1. Vegetative reproduction:
New plants develop from parts other than seed.
2. Agamospermy:
In this case embryo produce inside seed by abnormal process. It is two types
(a) Adventive Embryony:
Development of embryo directly from sporophytic tissue (2n) i.e, nucellus, integuments.
(b) Diplospory:
Megaspore mother cell without meiosis develops in to diploid embryo sac. Though diploid egg develop embryo without fertilisation. It is also known as diploid Parthenogenesis.
(c) Apospory:
Development of embryo sac directly from cell of nucellus. According to Panchanan Mahaswari; Apomix is maybe two types.
1. Recurrent Apomixis: it consist Vegetative propagation & agamospermy.
2. Non Recurrent Apomixis:
It is two types:
(a) Haploid Apogamy: Development of embryo from cells inside the embryo sac other than egg.
(b) Parthenogenesis: Development of embryo from egg without fertilisation.