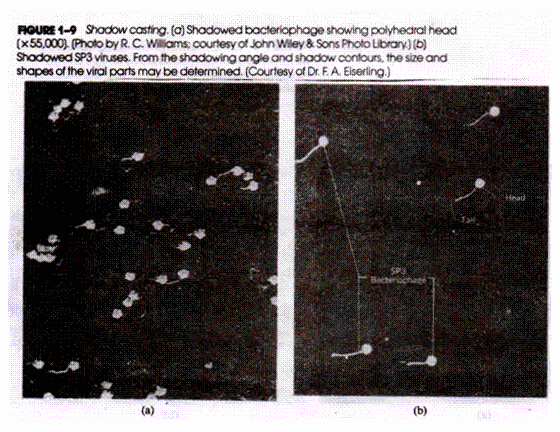
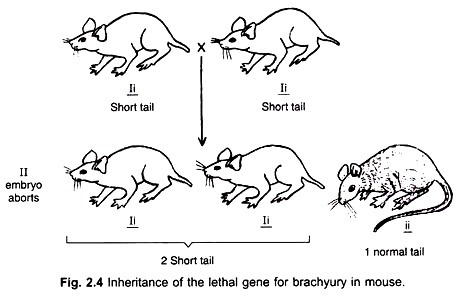
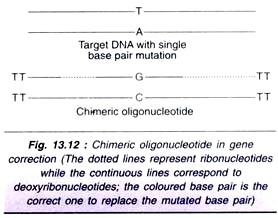
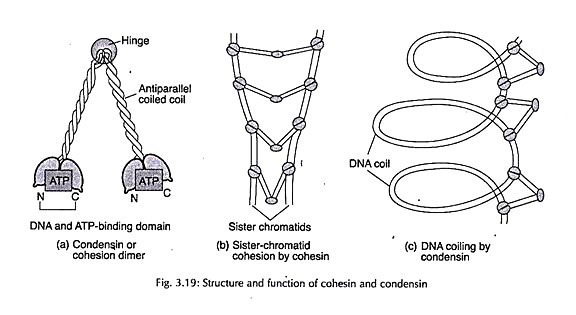
Let us make an in-depth study of the fertilization, embryology and seed of angiosperms. It is possible to get three types of seeds among both dicotyledons and monocotyledons according to the final equilibrium.
They are: (1) Exalbuminous Seeds (2) Albuminous Seeds and (3) Perisperm Seeds.
Pollination ends in a copious dusting of the stigma surface with pollen grains (Fig. 414). The next stage is the germination of the pollen to form a pollen tube wherein the male gametes are carried to the female gametophyte or the embryo sac whose development is already complete. Fertilization or syngamy or fecundation is the final phase of this biological process whereby the male and the female gametes fuse with one another giving rise to the embryo.
The pollen grain, after being liberated from the anther is viable for a short or long period. If it is transferred to the stigma during this period, it germinates. The germination of the pollen may be helped by the fluid of the stigma. Pollens may also be made to germinate in artificial culture media successfully. Germination may begin almost immediately after pollination as in sugarcane (Saccharum) Sorghum or it may take several hours or even days.
The pollen absorbs liquid from the moist surface of the stigma and the intine comes out through one of the germ pores in the form of a single unbranched tube (Fig. 409). Abnormally, however, several pollen tubes may develop out of the same pollen as in some Malvaceae, Cucurbitaceae, etc., or, the single pollen tube may be branched.
The pollen tube grows easily through the stigmatic papillae (Fig. 415) and then passes into the tissues of the style. The length of the stylar tissue which the pollen tube has to traverse may vary from nil in sessile stigmas to some 50 cm in the maize plants provided with silk (Fig. 416). Styles are solid or hollow.
The centre may be filled with a longitudinal transmitting tissue or, it may be a canal full of mucilage. The pollen tube may grow through this or through the tissue of the stylar wall. Usually, only the distal portion of a long pollen tube c6r»tains living protoplasm and, as the nuclei within it passes forward, callose plugs are left in the empty portions behind them.
When the pollen tube (now with the male gametes) reaches the ovary its course is to find out an ovule and to reach the embryo sac. The pollen tube usually enters the ovule (orthotropous or anatropous) through the micropyle. This is known as porogamy (Fig. 417). But, in some cases the pollen tube is found to enter through the chalaza. This latter method, known as chalazogamy (Fig. 418), is found in Casuarinaceae, Betulaceae, Fagaceae and other members of Archichlamydeae.
Formerly, much phylogenetic importance used to be attached to this character but, now it seems that the phenomenon is more of physiological importance as the same species may show both types of behaviour. In some cases (e.g., Cucurbita) the pollen tube enters the embryo sac piercing the integuments. This is termed mesogamy.
In the passage of the pollen tube towards the micropyle, it has to pass through some empty space. Sometimes the pollen tube is directed in its growth through this distance by an obturator which forms a sort of a plug on the micropyle and through which the pollen tube may grow. The obturator is usually of placental origin.
Fertilization:
On, penetrating the nucellus, whether through the micropyle, or, by a round about way through the chalaza, or, by piercing the integuments, the pollen tube penetrates the embryo sac and enters the egg apparatus. Ultimately, the tip of the pollen tube bursts and both the gametes are discharged. Probably the synergids play little or no role in this act. One of these gametes fuses with the egg cell of the egg apparatus.
The other gamete fuses with the secondary fusion nucleus. This behaviour of the two male gametes is termed double fertilization which was first observed by Nawaschin (1898) in Lilium and Fritilaria species. As a result of the first fertilization the oospore cell is formed which is the mother cell of the embryo and is a diploid cell containing 2n complement of chromosomes whereas the microspore and all nuclei of male and female gametophytes are haploid with n complement of chromosomes.
The secondary fusion nucleus of the embryo sac, however, normally becomes 2n at the time of the fusion undergone by it. (Exceptions to this have already been mentioned in connection with female gametophyte development). So, the fusion of this nucleus with the second male gamete is triple fusion and the resultant nucleus is triploid or 3n. This is the first nucleus of the endosperm.
Embryogeny and Endosperm Formation:
In the post-fertilizational changes within the ovule, the embryo and the endosperm are seen to develop simultaneously (Fig. 420). The oospore forms the embryo while the fertilized fusion nucleus (product of triple fusion) develops the endosperm. The other nuclei or cells within the embryo sac (synergids, antipodal cells) may behave differently but are destined to disorganise sooner or later.
Development of the Endosperm:
The Angiospermic endosperm, except in special cases, is a triploid (3n) tissue as it is a product of triple fusion involving double fertilization. It is, thus, distinct from the endosperm of Gymnosperms and heterosporous Pteridophytes where the endosperm is a simple haploid (n) tissue of the gametophyte not involving any complication like polar fusion or fertilization.
The real function of the endosperm is supplying nutrition to the growing embryo.
There are at least two families, Orchidaceae and Podostemonaceae, where the product of double fertilization soon disintegrates and endosperm development is completely suppressed.
In other plants, three types of endosperm development, nuclear, cellular and helobial, are usual:
(1) Nuclear Type:
This is the common type of endosperm development. Here, the endosperm nucleus gives rise to a number of free nuclei (Figs. 421 a, 424) which remain in the peripheral layer of embryo sac cytoplasm surrounding a large central vacuole. The nuclei, usually, become cut off by cell walls at a later stage if they be not already absorbed by the growing embryo. Such cell wall formation usually begins from the basal periphery.
The cells soon organise into an endosperm tissue and goes on increasing further. Development of the nuclear endosperm in Capsella bursa-pastoris (Fig. 424) is described later. In some cases the central vacuole may not be filled up even in the mature seed. This is seen in the palms. In coconut, the central cavity full of coconut water is the original embryo sac vacuole while the nuclei around it form the peripheral endosperm kernel.
(2) Cellular Type:
In a number of plants belonging to Annonaceae, Gentianaceae, etc., and in Adoxa, Peperomia, Villarsia (Fig. 421B), etc., division of the original endosperm nucleus is immediately followed by wall formation so that the endosperm is cellular from the beginning although some cells may enclose more than one nucleus.
(3) Helobial Type:
Among members of Helobiales (e.g., Vallisneria, Eremurus, Limnophyton, etc.) there is a type of endosperm development which is intermediate between the nuclear and the cellular types. Here, a partition wall develops between the two nuclei resulting from the first division of the endosperm nucleus so that the embryo sac is divided into two compartments.
A large number of free nuclei is now developed in the upper chamber while the lower nucleus forms a few of them or may not divide at all (Fig. 421C).
Development of the Embryo:
Side by side with the development of the endosperm, the zygote or oospore is developing the embryo. Both dicotyledons and monocotyledons begin embryo development in the same way but, there is considerable difference in later differentiation. In all Angiosperms the zygote (Fig. 422A) divides to develop a. two-celled proembryo (Fig. 422B). In most plants the first wall between the two cells is transverse while only in a few cases the first wall is more or less vertical (Piperad type, Fig. 423A1).
Of the two cells the one near the micropyle is termed the basal cell while the
one pointing towards the centre of the embryo sac is called the terminal cell. The basal cell usually forms the suspensor and may or may not contribute towards the future embryo in whose development the terminal cell takes the main part.
Johansen (1950) recognizes six types of embryo development among the Angiosperms according to the manner of differentiation of the basal and terminal cells: 
Although there is no essential difference between the embryogeny of monocotyledons and that of dicotyledons in the early stages, later differentiation makes the two embryos so different that the development of two such embryos are described separately below:
(1) Typical development of dicotyledonous embryo and endosperm:
The classical example of dicotyledonous embryo development is the plant Capsella bursa-pastoris of Cruciferae. The ovule is campylotropous so that the embryo sac and the later developed endosperm as well as embryo are horseshoe-shaped.
Moreover the micropyle being pointed downwards, the embryo looks upside down. Embryo development here is of the typical Onagrad or Crucifer type described above.
The oospore (Fig. 424A) divides by a transverse wall forming a large basal cell and a smaller terminal cell (Fig. 424B). The basal cell now divides transversely while the terminal cell divides by a vertical wall developing a │ -shaped 4-celled proembryo (Fig. 424B).
Next, the second basal cell quickly divides by a number of transverse walls giving rise to a row of cells called the suspensor (Fig. 424C). The lowest (micropylar) cell of the suspensor remains disproportionately large. As the suspensor increases in length, it pushes down the terminal embryonal cells deeper into the embryo sac. The embryonal cell, meanwhile, divides by three walls at right angles to one another giving rise to eight cells or octants (Fig. 424C).
This is called the embryonal mass. The lowest cell of the suspensor is called the hypo physis. The embryonal mass, along with the hypophysis, divides further (Fig. 424 D & E).
Ultimately, the four terminal octants form the two cotyledons, the four micropylar octants form the hypocotyl and the core of the radicle while the hypophysis forms the cortex and the epidermis of the radicle as well as the root-cap (Fig. 424 F & G). In the final stage (Fig. 424H) we find the mature embryo with the plumule developed also out of the four terminal octants. The suspensor gradually withers as the radicle is developed.
As the embryo is developing, the endosperm also keeps pace. Endosperm development, in this case, is strictly nuclear. The endosperm mother nucleus rapidly gives rise to a number of free nuclei which are arranged in the cytoplasm lining the periphery of the embryo sac while the centre is occupied by a big vacuole (Fig. 424D).
Gradually, cell wall formation begins from the periphery (Fig. 424F). The antipodal cells, in this, case, form a tissue which is short-living. The endosperm supplies nutrition to the embryo mainly through the suspensor. As the embryo of Capsella increases, the endosperm presses upon and sucks in plenty of food material from the nucellus which is soon consumed.
In the final stage (Fig. 424H) the nucellus has been completely consumed and most of the endosperm has also been consumed by the embryo. In the mature seed even this remnant of endosperm disappears so that the seed becomes exalbuminous.
(2) Typical Monocotyledonous Embryo:
There is no essential difference between the embryogeny of monocotyledons and that of dicotyledons. The endosperm develops in the same way but, as a monocotyledonous embryo develops a single cotyledon instead of two, there is some difference in later differentiation.
Luzula forsteri of Juncaceae may be taken as a type for monocotyledonous embryogeny. Here also the development is of the Onagrad or Crucifer type. The oospore divides by a transverse wall to form a large basal cell a and a’ smaller terminal cell b (Fig. 425A).
The terminal cell b divides into two by a longitudinal wall and the basal micropylar large cell a cuts off a cell a’ by a transverse wall developing a │ -shaped 4-celled proembryo (Fig. 425B).
The two cells b now divide into four quadrants by another wall at right angles to the first, the cell a’ divides into two by a longitudinal wall and the cell a cuts off a second cell a” by a transverse wall (Fig. 425C). The cell acts as the suspensor— while all the other cells take part in the formation of the embryo. The quadrants of b divide further giving rise to a tissue of which the lower half b (l) is destined to give rise to the lower part of the single cotyledon and the upper half b'(l’) to the upper part of the cotyledon, the hypocotyl with the core of the radicle and, later, the plumule.
The cell a’ on the top of it, forms a tissue which develops the upper part of the radical while a” develops the root-cap tip (Fig. 425 D, E, & F).
Apomixis:
Apomixis is a term coined by Winkler to signify any asexual method of propagation not involving the normal production of embryo by fertilization. This definition includes even propagation by bulbils. But, following the usual practice, here a number of cases are being discussed which involve seed or embryo formation without fertilization.
Parthenogenesis means development of an embryo directly from an egg cell or a male gamete. Apogamy means the development of a sporophyte (i.e., embryo) out of any gametophytic cell without the union of gametes. Apospory means the formation of a gametophyte (i.e., embryo sac or pollen) on a sporophyte without any reduction division.
Parthenogenesis, therefore, is a type of apogamy. Parthenogenesis of a normal haploid egg into an embryo has been noticed in Solanum nigrum, etc. This embryo and the resultant plant are haploid. This is termed haploid parthenogenesis. Haploid plants are usually sterile. Cases are also known (as in different species of Nicotiana and Crepis) where such embryos actually developed from male gametes. A second type of parthenogenesis, called diploid parthenogenesis, has also been noticed.
In these cases, the embryo sac develops from a diploid, i.e., sporophytic cell of the ovule without any reduction division (i.e., by apospory) so that the embryo sac and all cells within it are diploid. The diploid egg of this embryo sac develops a diploid embryo. This has been noticed in Taraxacum albidum and other plants.
Apogamy of other types also has been noticed. Any other haploid cell of a normal gametophyte (e.g., synergid or antipodal cell) may develop into an embryo. The resultant plant also is haploid as has been noticed in the orchid Orchis. On the other hand, if a synergid or an antipodal cell of a Taraxacum type embryo sac (i.e., diploid developed aposporously) develops into a diploid embryo, it will be a case of diploid apogamy.
Apospory, i.e., development of a gametophyte on the sporophyte without reduction division as seen in Taraxacum albidum has also been noticed in a few other plants like Eupatorium glandolusum, several species of Hieracium, etc.
An embryo may also develop directly from any diploid sporophytic cell (e.g., cells of nucellus, integument, etc.). This may be considered as vegetative growth of the Category of bulbils although they are not always so simple. This is sometimes called adventive embryony or sporophytic budding.
Polyembryony:
Polyembryony or the development of several embryos within the same ovule is quite common among Gymnosperms but rare among Angiosperms. This is often found in Citrus species (lemons and oranges —Fig. 426).
In these, the additional embryos are, usually, of nucellar origin, as discussed under adventive embryony above. Such additional embryos may also originate from the cleavage or splitting of the zygote (Cymbidium bicolor) or proembryo as seen in Erythronium americanum. Additional embryos are known to develop by epidermal budding of the first embryo (Nicotiana rustica) or from a filamentous proembryo (Nymphaea advena).
More commonly, two embryos are found in a seed of which one is normal, and the other is apomictic, the latter being usually haploid. Such haploid-diploid twin embryos are known in many cultivated plants like rice, linseed, wheat, potato, chilli, cotton, etc.
N.B. Johansen is of opinion that the term polyembryony should be strictly applied only when all the embryos are developed from the zygote, the proembryo, or by budding of the first embryo. The haploid-diploid embryos or such apomictic embryos are, thus excluded.
The Seed:
When the embryo is developed fully, the ovule or the megasporangium becomes the seed. With increase in the size of the ovule the integuments slowly dry up and form the protective seed coat, the outer integument forming the testa and the inner the tegmen.
Simultaneously, the internal organs also lose moisture and all food substances are transformed into insoluble reserve forms. Physiological activities are reduced to a minimum until the whole structure becomes dormant and almost dry when we call it seed. The seed is, therefore, a mature integumented megasporangium.
It has been seen that the developing seed contains a growing embryo within a growing endosperm, and the whole is contained within the nucellus. As the embryo increases, the endosperm decreases and the growth of both decreases the nucellus. Finally, an equilibrium is reached when seed development is complete.
According to the final equilibrium, it is possible to get three types of seeds among both dicotyledons and monocotyledons:
A. If the embryo completely fills up the ovule consuming both endosperm and nucellus, the seed is exalbuminous, there being no endosperm (Fig. 427). In such a seed food for the embryo is kept stored in the cotyledons.
B. If the endosperm be not completely consumed the embryo remains comparatively small and a large quantity of stored food (oily, mealy, horny, etc.) remains within the endosperm which the embryo could not consume but would consume in the germinating stage. Such a seed is albuminous (Fig. 428).
C. If even the nucellus is not consumed fully, it remains in the seed as a layer of perisperm (Fig. 429) over and above the endosperm and the embryo. This is the real nature of the perisperm as has been described in a number of monocotyledonous and dicotyledonous seeds.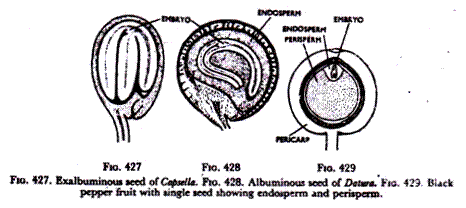
Aril and Caruncle:
Alter fertilization, an outgrowth looking like a third integument is sometimes seen to develop from the funicular (i.e., hilar) end. In litchi (Litchi chinensis) such a juicy fleshy outgrowth develops within the pericarp.
This (Fig. 430) is called the aril. In nutmeg (Myristica fragrans of Myristicaceae), such-a red-coloured, dry, leathery and branched aril (Fig. 431) forms the valuable spice ‘mace’ of commerce. There is a pulpy aril in Garcinia cowa and Garcinia mangostana.
In castor and some other Euphorbiaceae, a smaller outgrowth develops near the micropylar end. This is called the caruncle (Fig. 35). In pansy (Viola) such a caruncle develops at the hilum.