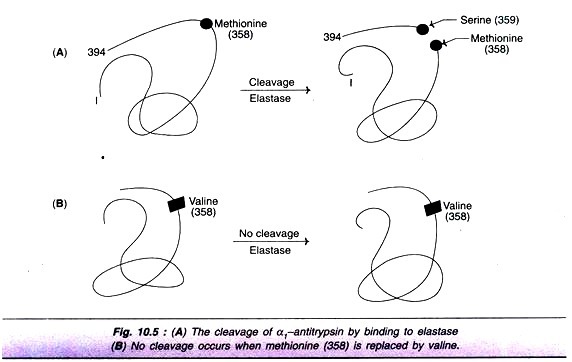
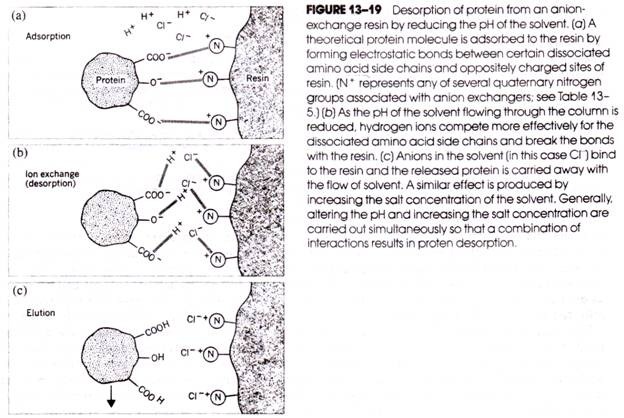
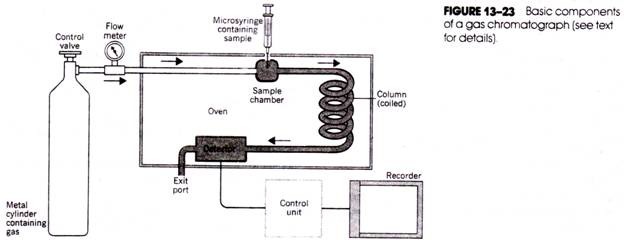
The following points highlight the two types of vitamins. The two types are: 1. Water Soluble Vitamins and 2. Fat Soluble Vitamins.
Vitamin Type # 1. Water Soluble Vitamins:
Thiamine:
Vitamin—B1:
Vitamin B1 is also called as Anti-Beri Beri vitamin and And-Neuritic vitamin.
Chemistry:
Thiamine is present as a crystal of thiamine hydrochloride. Chemically thiamine contains a pyrimidine ring and a thiazole ring linked by a methylene bridge. Both the rings are substituted.
The alcohol group on the thiazole ring is esterified with two phosphoric acid molecules to form thiamine pyrophosphate (TPP). Thiamine pyrophosphate is the active co-enzyme form of thiamine.
Source:
The richest sources of thiamine are rice bran, wheat bran, whole grains, nuts, germinating seeds, pulses, beans, lentils, yeast, liver, eggs, fish, meat and milk.
Daily requirement:
This vitamin cannot be stored in the body, as it is excreted in the urine, hence it should be provided daily in the diet. However the skeletal muscle can retain this vitamin for a short duration of time. Brain cannot retain any of the thiamine.
The daily requirement of thiamine depends upon the calorie requirement and the carbohydrate intake. For 3000 Kcal of energy 1.5 mg of thiamine is required per day. However—
i. For normal adult males the daily requirement is from 1.5 to 2.0 mg/day.
ii. For adult females the daily requirement is from 1.0 to 1.2 mg/day.
Assay methods:
Vitamin B1 is oxidized to thiochrome which can be assayed by fluorescence. Other biological and microbiological procedures are also available for its assay.
Metabolism:
Vitamin-B1 is freely absorbed by small intestine and drained into the liver. In the liver it is phosphorylated to thiamine pyrophosphate (TPP). This TPP coenzyme is present in all the tissues. There is no storage of this vitamin, hence regularly needed in the diet. About 10% of vitamin-B1 taken in the diet is normally excreted in the urine.
Physiological functions and biochemical role:
Thiamine is required for:
1. Utilization of carbohydrates in the body.
2. Maintenance of good appetite.
3. Normal brain metabolism.
Thiamine in its active coenzyme form i.e. as thiamine pyrophosphate and Mg function as coenzyme for (1) Oxidative decarboxylation reactions and (2) Transketolation reactions. Ex.
For oxidative decarboxylation’s:
Deficiency diseases:
Thiamine deficiency results in impaired utilization of carbohydrates due to which pyruvate and lactate accumulates in the cells. But this impairment is not uniform; the brain cells are more affected than the skeletal muscle. Blood brain barrier becomes permeable to pyruvate in rats. Erythrocyte transketolase levels are lower then normal.
The symptoms of thiamine deficiency are loss of appetite (anorexia). The clinical condition of thiamine deficiency is known as ‘Beri-Beri’, characterized by polyneuritis (various defects of nervous system), edema, cardio-vascular changes, weakness, muscular atrophy, headache, insomnia, gastro-intestinal disorders etc. Wernicke’s encephalopathy or acute thiamine deficiency is seen in alcoholics.
Beri-Beri is of four types viz.:
(a) Dry Beri-Beri:
In this nervous symptoms or polyneuritis predominates.
(b) Wet Beri-Beri:
In this the symptoms are associated with edema and serous effusions.
(c) Acute pernicious Beri-Beri:
In this the symptoms of heart are involved.
(d) Mixed Beri-Beri:
All the above symptoms.
Another type of Beri-Beri is infantile Beri-Beri seen in breast fed children, whose mothers’ milk is deficient in thiamine.
Antagonists:
Pyrithiamine, oxythiamine and 2-n-butyl thiamine are the antagonists of thiamine. Foxes develop a type of paralysis called ‘chastek paralysis’ when they eat raw fish. It is caused by the presence of enzyme “thiaminase” in raw fish which destroys thiamine.
Riboflavin:
Chemistry:
Riboflavin is made up of substituted isoalloxazine ring. To this ring ribityl alcohol is attached.
The ribityl alcohol of the riboflavin is phosphorylated to form riboflavin phosphate or flavin mononucleotide (FMN).
Riboflavin is also linked to an adenosine nucleotide through a pyrophosphate linkage to form flavin adenine dinucleotide (FAD).
FMN and FAD are the two active coenzyme forms of riboflavin.
Source:
Riboflavin is rich in animal sources like milk, liver, kidney, heart, egg yolk and in sprouts.
Daily requirement:
The daily requirement of riboflavin depends upon the calorie requirement of an individual. For 1000 cal of energy 0.5 mg of riboflavin is recommended. On an average 1.5-2.0 mg/day of riboflavin is recommended.
Physiological functions of riboflavin:
1. It is required for the regulatory functions of some hormones concerned with carbohydrate metabolism.
2. It stimulates the optic nerve in presence of light.
Coenzyme activities:
FAD and FMN act as coenzyme for some enzymes called flavor proteins. They help in the oxidation-reduction reactions of cell metabolism. The hydrogen’s are transported by reversible reduction of the coenzyme by two hydrogen atoms added to the nitrogen (N) at positions 1 and 10.
The enzyme reactions catalysed are as follows:
Deficiency diseases:
(1) Characteristic lesions of the lips.
(2) Fissures at the angles of the mouth (cheilosis).
(3) Dermatitis of the face.
(4) Magenta tongue.
(5) Certain functional and organic disorders of the eye.
Antagonists:
Dichlororiboflavin and isoriboflavin.
Niacin:
Chemistry:
The chemical name of niacin is nicotinic acid. It contains a pyridine ring with a carboxylic acid.
Nicotinic acid is converted to its amide form called niacin amide or nicotinamide.
Nicotinamide combines with a ribose phosphate and later on gets esterified to an adenine nucleotide to form the ‘Nicotinamide adenine dinucleotide (NAD+)
The ribose sugar of adenine may be phosphorylated to form ‘Nicotinamide adenine dinucleotide phosphate (NADP+)’. NAD+ and NADP+ are the two coenzyme forms of niacin.
Source:
Liver, fish, beans and peanuts. Niacin can also be formed in the human body form the amino acid – tryptophan. 60 mg of tryptophan can give rise to 1 mg of niacin.
Daily requirement:
15-20 mg/day. Niacin requirements depend upon the quality and quantity of protein in the diet, as niacin can be formed from tryptophan.
Functions:
Niacin in the form of NAD+ and NADP+ acts as coenzyme for a number of oxidoreductases. They act as electron acceptors during the enzymatic removal of hydrogen atoms.
NAD acts as coenzyme for dehydrogenases in oxidation of various food stuffs.
NADPH+ acts as coenzyme for reductases in the synthesis of fatty acids and cholesterol.
Functions of NAD+ as coenzyme:
Functions of NADP as coenzyme (its formation and utilization):
Deficiency diseases:
The deficiency disease of niacin is pellagra. It is characterized by “3 Ds” i.e. Dermatitis, Diarrhoea and Dementia (headache, forgetfulness, depression, anxiety, etc.). If not treated it results in 4th ‘D’ i.e. Death.
Pyridoxine:
Vitamin – B6:
Chemistry:
The chemical name of pyridoxine is 2-methyl-3-hydroxy-4, 5-di-hydroxymethyl pyridine. The alcohol on 4th position may be oxidized to an aldehyde to form pyridoxal or it may be substituted by an amino group to form pyridoxamine. Hence vitamin B6 exists in three forms i.e., pyridoxine, pyridoxal and pyridoxamine.
The – OH group at the 5th position may be phosphorylated to give pyridoxal phosphate and pyridoxamine phosphate. These are the two active coenzyme forms of this vitamin.
Source:
Yeast, rice polishing, milk, meat, eggs, leafy vegetables and liver. Intestinal bacteria can also synthesize this vitamin.
Daily requirement:
1.6-2.0 mg/day.
Functions:
1. Pyridoxine is essential for the growth of infants.
2. Pyridoxal phosphate acts as coenzyme for—
(a) Transamination reactions—takes up amino group to form pyridoxamine phosphate.
(b) Decarboxylation—in the formation of dopamine (GABA).
(c) Dehydration.
(d) Desulphuration.
(e) Deamination.
(f) Trans-sulphuration.
(g) Kynureninase enzyme in niacin synthesis.
(h) Synthesis of serotonin, catecholamine’s and heme.
A Schiff’s base is formed as an intermediate in the reactions catalysed by pyridoxal phosphate.
Deficiency diseases:
Vitamin B6 deficiency is rare. However irritability, depression, peripheral neuropathy, hypochromic microcytic anemia, cystathionuria, etc. may be seen.
Antagonists:
Isonicodnic acid hydrazide (INH) and hydrazoline. Infants when treated with INH for tuberculosis suffer form convulsions because of structural resemblance of INH with vitamin B6.
Pantothenic Acid:
Chemistry:
Pantothenic acid contains β-alanine and pantoic acid joined by a peptide bond.
The active coenzyme form of pantothenic acid is coenzyme-A. Coenzyme-A is formed by combination of pantothenic acid with adenine, ribose, phosphoric acid (Adenine nucleotide) and β-mercaptoethylamine.
Source:
Pantothenic in Greek means ‘from everywhere’. It is so named because it is present in all the common food sources. Its richest source is honey. The intestinal flora also supplies considerable amount of this vitamin.
Food source:
Cereals, nuts, oil seeds, egg, liver, wheat grain, legumes, rice polishing, milk, meat and fish are good sources of this vitamin. Vegetables like potato have relatively less amount of this vitamin.
Functions:
1. Pantothenic acid is required for the growth of infants and children.
2. Coenzyme-A is required for the activation of acetate to acetyl-CoA.
3. Co-A is required to form succinyl-CoA for the citric acid cycle to operate normally.
4. Coenzyme-A is required for the activation of fatty acids for their oxidation.
5. Coenzyme-A is also required for fatty acid and cholesterol synthesis.
Deficiency diseases:
As this vitamin is widely distributed, the deficiency rarely occurs. However nausea, vomiting, G.I tract disorders, irritability, inadequate growth, anemia, fatty liver, etc. may occur due to its deficiency. Impaired growth and reproduction, loss of hair, sensitivity to insulin, burning feet syndrome are other deficiency manifestations of this vitamin.
Biotin:
Chemistry:
It is a heterocyclic compound containing sulphur and a valeric acid side chain. It is attached to lysine residue of the enzyme through the valeric acid to form the active coenzyme i.e., biocytin.
Source:
Egg, liver, fish, meat, beans, germinating seeds, intestinal flora, and peanut. Richest source is → honey. Poor source is → fruits and vegetables.
Daily requirement:
Only if the intestinal flora is disturbed, the requirement of biotin ranges form 100-300 (µg/day).
Functions:
Biotin is involved in CO2 transfer and CO2 fixation. Ex.
Deficiency diseases:
Only if raw egg white which contains avidin is taken, it binds and inhibits the absorption of biotin that leads to deficiency. Signs include—alopecia, greying of hair and nervous symptoms.
Folic Acid:
Chemistry:
There are three components in folic acid viz:
(1) Pteridine
(2) Para amino benzoic acid (PABA) and
(3) Glutamic acid.
Pteridine nucleus comprises of pyrimidine and pyrazine rings. Further pteridine and PABA are collectively called pteroic acid. All the components are together known as pteroyl glutamic acid (PGA) i.e. folic acid.
Folic acid is readily absorbed by the intestine and not stored but undergoes reduction in the liver by the enzyme folic acid reductase that reduces folic acid to tetrahydrofolic acid (THF). This is the active coenzyme form of folic acid. This reaction requires NADPH+ and ascorbic acid.
Functions:
The main function of folic acid is the transfer of one carbon units (moiety or fragments).
The various one carbon moieties transferred by THF are:
1. —CH3 = Methyl group
2. —CH2OH = Hydroxy methyl group
3. —CH2– = Methylene
4. —CHO = Formyl
5. —CH = Methylidine or methenyl
6. —COOH = Carboxyl
7. —C — O = Carbonyl
8. —CH=NH = Formimino
These one carbon moieties get attached either to the ‘N’ at position 5 or to the ‘N’ at position 10. They can get attached both to 5th nitrogen and 10th nitrogen. If the one carbon (1-C) moiety is attached to the 5 nitrogen of folic acid then it is known as N – (1-C)-THF. If the one carbon moiety is attached to the 10th nitrogen then it is N10-(1-C)-THF. If the 1-carbon moiety is linked to both 5 nitrogen then it is called N5-N10-(1-C)-THF.
Some examples:
1. N5-Methyl tetrahydrofolate
2. N5, N10-Methylene tetrahydrofolate
3. N5-Formyl tetrahydrofolate
The N and N10 forms are inter-convertible. The one carbon moieties are derived from several sources and utilized to form several compounds.
Action of THF:
Source:
The microorganisms of the intestinal tract can synthesize folic acid in considerable amounts. These microorganisms need PABA to synthesize this vitamin. Therefore if PABA is supplied in sufficient quantities the requirement of folic acid can be fulfilled. During antibiotic therapy the micro flora die leading to deficiency of folic acid hence the exogenous food sources of folic acid like liver, eggs and leafy vegetables are required in the diet.
Daily requirement:
As adult human liver can store to about 5-20 mg of folic acid the daily requirement of folic acid is about 300-400 µg, which can be easily supplied by the intestinal flora.
Deficiency disease:
During treatment with sulphonamide drugs there will be deficiency of folic acid as the sulphonamide drugs competitively inhibit the synthesis of folic acid by the microorganisms. This competition is as a result of structural similarity between the drug and PABA.
The deficiency results in poor synthesis of the pyrimidine thymine which in turn affects DNA synthesis, hence affects cell division. The signs of deficiency are magaloblastic anemia, glossitis and G.I tract disturbances and leucopenia.
Antagonists:
Aminopterin, amethopterin, trimethoprim and methotrexate.
Lipoic Acid:
Chemistry:
It is a sulfur containing fatty acid named 6, 8-dithiooctonoic acid. Dihydrolipoic acid is the reduced form of lipoic acid and is the active coenzyme form.
Biochemical functions:
1. It is an essential cofactor for many enzyme complexes in aerobic metabolism, specifically the pyruvate dehydrogenase complex.
2. Other enzymes that use lipoic acid are 2-oxoglutarate dehydrogenase (OGDH) complex, branched chain oxoacid dehydrogenase (BCDH) complex, glycine cleavage complexes (CtCY) and acetoin dehydrogenase (ADH) complex. Lipoate participates in transfer of acyl or methylamine groups in 2-oxoacid dehydrogenase (2-OADH) and glycine cleavage complexes (GCV), respectively.
3. Lipoic acid is an effective antioxidant and thus it prevents the symptoms of vitamin C and vitamin E deficiency. Dihydrolipoic acid is able to regenerate (reduce) antioxidants, such as glutathione, vitamin C and vitamin E, maintaining a healthy cellular redox state.
4. It increases the cellular uptake of glucose by recruiting the glucose transporter ‘GLUT4’ to the cell membrane.
5. It acts as a good chelating agent for mercury.
Clinical uses:
i. Intravenous administration of alpha lipoic acid (ALA) to people with acute and severe liver damage results in recovery of full liver function. Hence it is used successfully for the treatment of chronic liver disease (viral hepatitis, autoimmune hepatitis, etc.).
ii. Use of ALA with various oral antioxidants results in the long term survival of patients with metastatic pancreatic cancer.
iii. Alpha lipoic acid has the ability to modify gene expression by stabilizing NF kappa B transcription factor, hence ALA is used for the treatment of various cancers for which no effective treatments exist.
iv. Intravenous ALA completely reverses the signs and symptoms of B-cell lymphoma. Its enhancement of glucose uptake by cells favours its use in diabetes.
v. The use of carnitine and lipoic acid results in improved memory performance and delayed structural mitochondrial decay. As a result, it may be helpful for people with Alzheimer’s disease or Parkinson’s disease.
vi. It is used as a chelating agent in treatment of mercury intoxication. It is particularly suited to this purpose as it can penetrate both the blood-brain barrier and the cell membrane. Other chelators such as dimercaptosuccinic acid (DMSA) and 2, 3-dimercapto-1-propanesulfonic acid (DMPS) are unable to cross the brain-blood barrier and to remove mercury from the brain.
Food sources:
Lipoic acid is found in a variety of foods, notably kidney, heart and liver meats as well as spinach, broccoli and potatoes.
Dietary requirement: 60 mg/kg bw/day.
Vitamin-B12:
Cobalamine:
Chemistry:
1. It has four pyrrole rings — I, II, III and IV.
2. Three rings are linked by methylene bridges.
3. Ist and IVth rings are linked directly.
4. The tetrapyrrole ring structure of vitamin B19 is known as ‘corrin’ ring system.
5. Cobalt atom is present in the centre linked by coordinate linkages to the nitrogen’s of the pyrrole rings.
6. The cobalt is also linked to cyanide. Hence it is called cyanocobalamine.
7. Cobalt is even linked to a ribonucleotide having the base 5,6-dimethylbenzimidazole.
8. The side chains of the corrin ring are much longer.
9. The side chain of the IVth ring is linked to the ribonucleodde.
Metabolism:
Vitamin B12 is known as the ‘extrinsic factor of castle’. Absorption of vitamin B12 requires an ‘intrinsic factor of castle’ secreted by the gastric glands. It is a glycoprotein and helps in the absorption of vitamin B12 from the intestine along with a releasing factor. B12 is transported in the plasma bound to specific carrier proteins called transcorrin-I and transcorrin-II.
The active coenzyme form of vitamin B12 is known as cob-amide coenzyme.
Functions:
The coenzyme activity of cyanocobalamine is that of 1, 2-hydrogen shift and methyl group transfer.
Folic acid takes up the methyl group from the methyl donor and donates it to cobalamine and folic acid itself goes to folate pool to accept another methyl group. This is known as folate cycle.
1, Vitamin B12 is essential for the normal maturation and development of erythrocytes.
2. It is necessary for the synthesis of DNA.
3. It is required for the conversion of methyl malonyl-CoA to succinyl-CoA.
4. Methylation of homocysteine to methionine.
5. Inter conversion of glutamic acid and P-methyl aspartic acid.
Source:
Neither plants nor animals can synthesize vitamin B12. Only certain microorganisms can synthesize it. However as B12 is stored in the organs of various animals the sources of B12 are liver, egg, meat and fish.
Daily requirement:
5 µg/day.
Deficiency diseases:
Megaloblastic anemia (pernicious anemia), demyelinadon, neurological lesions and infertility.
Vitamin-C:
Ascorbic Acid:
Chemistry:
It is a strong reducing substance. The structure resembles a hexose sugar. It is easily oxidized to dehydroascorbic acid.
Functions:
It is concerned with the metabolism of connective tissue, particularly of collagen.
1. It maintains the redox potential of the cell.
2. Proline is converted to hydroxyproline in presence of vitamin C. Hydroxyproline is an important constituent of collagen.
3. It helps in the absorption of Iron from the intestine.
4. High doses of vitamin-C in the diet, reduces the duration and severity of common cold.
Source:
The citrus fruits (lemons and oranges) are the richest sources. Other sources are fresh green vegetables, cabbage, lettuce, guavas, berries, melons and tomatoes.
Daily requirement:
30 mg for infants and 70 mg for adults.
Deficiency diseases:
Scurvy is the deficiency disease of vitamin C. The main defect is poor deposition of intercellular cement substance (i.e. collagen). The capillaries are fragile and so there is a tendency to hemorrhage. Wound healing is delayed due to deficiency in the formation of collagen. Gums are swollen and decay easily. There is poor dentine formation and tooth loss. Weak bones leading to fracture.
Vitamin Type # 2. Fat Soluble Vitamins:
Vitamins insoluble in water but soluble in fats or fat solvents are known as fat soluble vitamins. The fat soluble vitamins are vitamin A, D, E and K.
Vitamin–A:
Chemistry:
Vitamin A functions in the human body in four different forms-
1. Vitamin A1 known as Retinal—the aldehydic form
2. Vitamin A2 known as Retinol—the alcoholic form
3. Vitamin A3 known as Retinoic acid—the acid form
4. Vitamin A4 known as Retinyl acetate—the acetate form
Vitamin A, as such, is neither synthesized by the animals nor by the plants. Instead, plants synthesize the pro-vitamins of vitamin A known as carotenoids that are converted to the active vitamin in the animal body. There are many carotenoids that includes α, β, γ carotenes and others. Among the various carotenoids; β-carotene is the most potent precursor of vitamin A.
β-Carotene is made up of two β-ionone rings connected through an eighteen member hydrocarbon chain, substituted by methyl groups at a few points.
β-Carotene:
The pro-vitamins (β-carotene) are converted into vitamin A in the intestinal wall in animals but in man this transformation takes place in liver. There is an enzyme called β-carotene 15, 15′-oxygenase in the liver of man and intestinal wall of other animals that cleaves β-carotene at the central position releasing two molecules of active vitamin A.
This reaction takes place in presence of α-tocopherol (vitamin E) and it is a dioxygenase reaction in which molecular oxygen reacts with the 2 central carbon atoms of beta- carotene followed by cleavage of its central double bond to yield 2 molecules of vitamin A aldehyde (retinal). Vitamin A alcohol is then produced by reduction of the aldehyde in a NADH-dependent reaction catalysed by retinene reductase.
Absorption and transport:
The dietary sources of vitamin A to the humans and animals are via the conversion of beta-carotenes (plant sources) to vitamin A and hydrolysis of retinyl esters (animal sources) to retinol in the intestine. Retinol is absorbed in the cell membrane of the intestine, re-esterified inside the cell of the intestine and finally absorbed via the lymph. A significant amount of vitamin A is also absorbed directly into the blood circulation along with the other dietary fats that form chylomicrons. Vitamin A is stored in the liver as palmitate esters.
Vitamin A is transported from the storage organ (liver) to the organs of utilization (eye, skin etc.) being bound to retinol-binding protein (RBP) which has a molecular weight of about 20,000 Da. It is also transported being bound to pre-albumin in the blood.
Biochemical Functions:
1. It is required for the normal vision and general growth of the body.
2. It accelerates the development of the nervous system and bones.
3. It maintains the structural integrity of the cell membrane and membranes of lysosomes and mitochondria. Thus it keeps the skin, kidney and other organs intact thereby preventing their degeneration.
4. It enhances the carbohydrate metabolism especially gluconeogenesis from lactate, acetate and glycerol.
5. It is also involved in mucopolysaccharide biosynthesis.
6. It enhances protein synthesis by activating aminoacyl-tRNA synthetizes.
7. It accelerates the transcription and translation process in the cell.
8. It is also required for DNA metabolism.
Vitamin – A and vision (Rhodopsin cycle):
There are two types of cells in the retina of the eye viz. rods (for dim light vision) and cones (for bright light vision). The rod cells contain rhodopsin (retinal pigment or visual purple). When light strikes the retina, rhodopsin is split into its protein component; opsin and the non-protein; retinene (all trans-retinal).
In the eye, the trans-retinal is reduced to trans retinol by the enzyme retinene reductase and NADH. The trans-retinol is inactive in the synthesis of rhodopsin and hence it passes into the blood, where it isomerizes into cis-retinol.
In the dark or dim light the active cis-retinol enters the retina from the blood where it is oxidized to cis-retinal by the reverse action of retinene reductase and NAD. Now the cis-retinal combines with the protein opsin to give back rhodopsin and thus the cycle is repeated thereby helping in the normal vision of the eye.
Deficiency diseases:
1. Nyctalopia—night blindness
2. Xeropthalmia—complete blindness
3. Colour blindness
4. Keratomalacia—Dryness of eye, skin and keratinization of respiratory, intestinal, urinary tracts, salivary glands and the genital system.
Hypervitaminosis:
Nausea, vomiting and headache are the common symptoms of vitamin A excess.
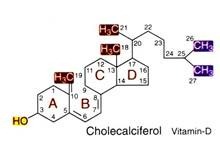
Vitamin–D:
Chemistry:
Vitamin-D is chemically known as calciferol. It is commonly known as antiricketic vitamin. It is a fat-soluble vitamin and exists in two forms—(1) vitamin D2 (ergocalciferol) and (2) vitamin D3 (cholecalciferol). Cholecaliferol is the most prominent form of vitamin D in humans. Vitamin D is similar to the classic steroid hormones. It is derived from cholesterol.
Daily requirement and source:
The daily requirement of vitamin D is 100 units. Lactating women need 400 units/day. The rich dietary sources of vitamin D are cod liver oil, fish liver oil, egg yolk, milk and animal liver. Vitamin D3 can be produced photo chemically by the action of sunlight or ultraviolet light from the sterol precursor, 7-dehydrocholesterol which is present in the epidermis or skin.
Vitamin D3 can be endogenously produced and as long as an individual has access on a regular basis to sunlight there is no dietary requirement.
Absorption:
The dietary vitamin D is absorbed from the upper part of small intestine and requires bile salts. Some of it is also absorbed along with other dietary fats through the chylomicrons. It is also absorbed by the skin if cod liver oil is massaged over the skin. Vitamin D formed by the ultraviolet radiation is also absorbed through the skin.
Metabolism:
Cholesterol is dehydrated at the 7th position to 7-dehydrocholesterol. In the skin, photo conversion of 7-dehydrocholesterol to cholecalciferol (vitamin D3) takes place. Cholecalciferol is metabolised with the help of the enzyme D3-25-hydroxylase in the liver to 25-hydroxycholecalciferol (25(OH)D3), which is the major form of vitamin D circulating in the blood compartment. This is further metabolized in the kidney by the enzyme 25 (OH) D3-l-hydroxylase (mediated by parathormone) to 1, 25- Dihydroxycholecalciferol, this is the active form of vitamin D.
Plasma vitamin D binding protein (DBP) carries vitamin D3 and all of its metabolites to their various target organs through the blood. The production of 1, 25(OH)2D3 is modulated according to the calcium and other endocrine needs of the body. The chief regulatory factors are 1,25(OH)2D3 itself, parathyroid hormone (PTH), and the serum concentrations of calcium and phosphate.
Probably the most important determinant of the 1-hydroxylase is the vitamin D status of the individual. When circulating concentrations of 1,25(OH)2D3 are low, production of 1,25(OH)2D3 by the kidney is high, and when circulating concentrations of l,25(OH)2D3 are high, the output of 1,25(OH)2D3 by the kidney is sharply reduced.
Biochemical functions:
Vitamin D has three different sites of action i.e. intestine, bones and kidneys. The primary biochemical action of vitamin D is to regulate blood calcium.
This is brought about by the following mechanisms:
1. Vitamin D increases the absorption of calcium and phosphorus from the intestine by decreasing the pH.
2. It increases the biosynthesis of calcium binding protein in the intestinal mucosal cells that helps in the transport of calcium through the intestine.
3. It reduces the excretion of calcium and phosphorus from the kidneys with the help of parathyroid hormone, thereby increasing the blood calcium levels.
4. When the serum calcium reduces, it promotes the mobilization of calcium from bones and releases it into the blood.
5. It aids in mineralization of bones (collagen).
6. It increases the citrate level of blood, bone, kidney and heart tissues and also excretion of citric acid.
7. It stimulates the activity of phytase which catalyses the hydrolysis of phytic acid in the intestine.
Deficiency diseases:
Human clinical disorders related to vitamin D can be considered as those arising because of:
i. Altered availability of vitamin D
ii. Altered conversion of vitamin D3 to 25(OH)D3 and then to 1,25(OH)2D3.
iii. Variations in end organ responsiveness to 1,25(OH)2D3 or possibly 25(OH)2D3.
There are two major clinical disorders related to vitamin D deficiency, they are:
(1) Rickets:
Bowed legs.
(2) Osteomalacia:
Soft bones with tendency to break easily.
Hypervitaminosis:
Nausea, vomition and headache are the common symptoms of vitamin D excess.
Vitamin-E:
Introduction:
Vitamin E is the anti-sterility factor. It is necessary for fertility of male and the birth process in the female, therefore it is called tocopherol (Greek word Tokos — child birth, Pheros — to bear and ol = alcohol).
Chemistry:
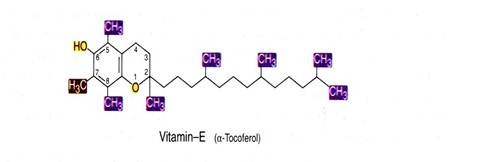
Vitamin E is chemically known as a-tocopherol. It is an oily substance, heat stable, readily oxidized and acts as powerful antioxidant. Owing to its antioxidant property it protects other vitamins like vitamin A from oxidation.
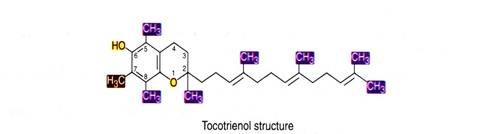
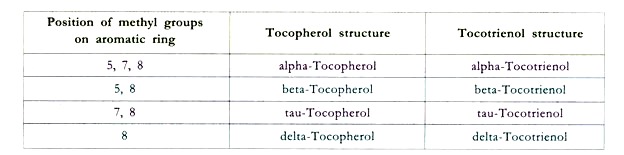
There are many derivatives of this vitamin viz. α, β, γ and δ due to the presence of different substituents on the aromatic ring at positions 5, 6, 7, and 8. α-tocopherol is 5,7,8- trimethyl derivative and has the highest vitamin activity. Vitamin E has a characteristic double ring structure called the chomane ring.
The tocotrienols share the same ring structure, but have an unsaturated tail.
Absorption and storage:
Vitamin E is readily absorbed along with fat from the GI tract. It is metabolized to unidentified substances. It is absorbed to a great extent from salt solution. Bile is essential for vitamin E absorption, since bile contains bile salts which emulsify the fat by lowering the surface tension and theory favouring its absorption. It is stored in the liver, muscle and body fat stores.
Sources:
Meat, liver, fish, chicken, vegetable oils, particularly wheat germ oil, corn oil, cotton seed oil and safflower oil are rich sources. Others are green leafy vegetables like spinach and lettuce and egg yolk.
Biochemical role:
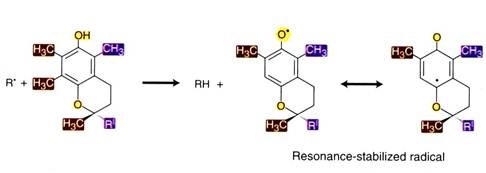
It has antioxidant activity. Tocopherols (vitamin E) can interrupt free radical chain reactions by capturing the free radical. This imparts to them their antioxidant properties. The free hydroxyl group on the aromatic ring is responsible for the antioxidant properties. The hydrogen from this group is donated to the free radical, resulting in a relatively stable free radical form of the vitamin.
Polyunsaturated fatty acids (constituent of cell membranes) are easily attacked by molecular oxygen resulting in the formation of peroxides. The tocopherols prevent this. Vitamin E and other antioxidants such as vitamin C, selenium, sulphur containing amino acids (cystine and methionine), ubiquinone, vitamin A and carotenes prevent lung tissue damage from atmospheric ozone and nitrogen dioxide. Tocopherols prevent oxidation of vitamin A. It prevents enzymes in muscles, nerves and gonads from destruction. It also prevents the development of cerebral disorder. It is involved in heme synthesis.
Thus the physiological role of vitamin E can be summarized as under:
1. It prevents peroxidation of polyunsaturated fatty acids in tissues and membranes.
2. It prevents haemolysis of erythrocytes by oxidizing agents like H2O2 and dialuric acid.
3. It prevents the degeneration of cellular and subcellular membranes rich in polyunsaturated fatty acids (PUFA).
4. It prevents poisoning of liver cells. The liver is exposed to carbon-tetrachloride, chloroform and other toxic chemicals.
5. It prevents demyelination of nerve fibres and prevents distortion of the axis of the nerves in the spinal cord.
6. Respiration in the mitochondria is depended upon the availability of vitamin E and the activators present in microsomal supernatant extraction of the cells also need this vitamin.
7. Requirement of vitamin E is dependent on the amount of PUFA in diet and selenium status in the body. It spares the activity of selenium present in traces in the body.
8. It prevents hepatic necrosis produced by diet deficient in sulphur containing amino acids.
9. Its action as an antioxidant prevents rancidity.
10. It is important for reproductive physiology. In male rats that are deprived of vitamin E, the seminiferous epithelium undergoes irreversible degeneration and permanent sterility occurs. In females the deficiency of vitamin E does not affect the ovary. Ovulation, conception and implantation take place normally, but foetus dies in the uterus a few weeks after conception and undergoes resorption.
Deficiency diseases:
1. It causes discolouration of the enamel of the teeth due to oxidation of unsaturated fatty acids present in these structures to peroxide.
2. Anaemia is caused in monkeys due to lack of hemophysins in bone marrow, rather than by destruction of RBC.
3. Increased fragility of RBC.
4. Thrombocytosis and edema.
5. Permanent sterility in males and death of foetus in uterus after few weeks of implantation in females.
6. Necrosis of hepatic cells.
Daily requirement:
Adults-10 mg/day. If PUFA in the diet is 1 gm./day then the requirement of vitamin E is as high as 35 gms/day. Pregnant or lactating women require greater amount of vitamin E.
Hypervitaminosis:
Leads to nausea.
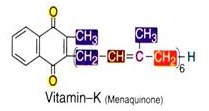
Vitamin-K:
It is chemically known as menaquinone (i.e. K3) whereas K1 and K2 are napthaquinones (present in plants). Menaquinone is an anti-hemorrhagic vitamin i.e. it prevents hemorrhages by activating the
Sources:
Green leafy vegetables like cabbage, spinach etc. predominantly contain vitamin K, and are the best sources. Cauliflower, soya-bean, wheat-germ etc.. have proved to be good sources of this vitamin. Tops of carrots contain considerable amounts. Animal products contain very little although milk and eggs contain small amounts. Vitamin K2 is produced by most bacteria present in the human intestine if it is not supplied in the diet.
Biochemical role:
Vitamin K is necessary for proper formation of prothrombin (the blood plasma protein), the inactive precursor of thrombin which is an enzyme that converts the protein fibrinogen (of blood plasma) into fibrin, the insoluble fibrous protein that holds blood clots together.
1. Vitamin K increases the activity of many clotting factors.
2. It initiates the biosynthesis of the enzyme proconvertin of the liver cells which catalyses the formation of prothrombin (precursor of thrombin protein).
3. It takes part in election transport chain.
4. It acts as a coenzyme for carboxylation of glutamate to y-carboxyglutamate.
Deficiency conditions:
Increased clotting time and decreased blood prothrombin levels are seen in vitamin K deficiency. There is continuous bleeding specially during delivery of the fetus.
Daily requirement:
The average diet contains adequate amount of the vitamin being synthesized by the bacteria present in the intestine. Hence deficiency symptoms are not seen in healthy individuals except in new born infant fed on mother’s milk when the mother’s diet has low vitamin K content.
Hypervitaminosis:
Very large doses of vitamin K are toxic.