Sulfonamides are synthetic chemotherapeutic agents. They were in common use as antimicrobial drugs prior to the advent of antibiotics. Frequent development of cross drug resistance in bacteria isolated from animals has now-a-days reduced their clinical values.
Recently their use in combination with trimethoprim or orimethoprim is favoured on account of synergistic action and prevention of emergence of resistant bacteria.
In 1935, Domagk, a German scientist demonstrated the antibacterial therapeutic property of Prontosil-an azo dye possessing p-amino-benzene sulfonamide group. He was awarded Nobel Prize of Medicine in 1939 for his outstanding contribution. The antibacterial activity of the drug was due to its sulfanilamide component.
Chemistry:
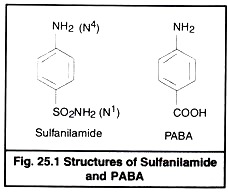
Chemically sulfa drugs are amphoteric. They behave as weak organic acid with pKa 4.79 to 8.56. Though they are weakly soluble in water, their solubility is increased at alkaline pH. Sodium salts are however easily soluble in water. The sulfacetamide is neutral in pH and is used to combat eye infections. The basic structure of sulfanilamide and PABA is given in Fig 25.1.
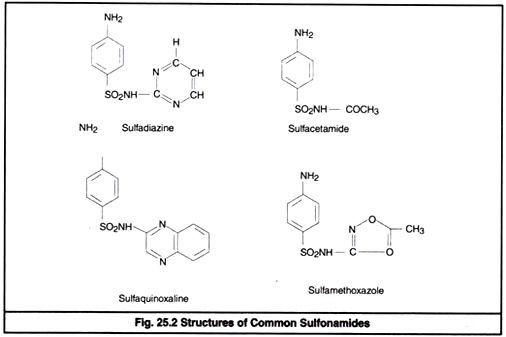
The nitrogen of amino group at para position is designated as N4 while nitrogen of So2 NH2 is designated as N1. Systemic sulfa drugs are evolved by substitution at N1 position whereas gut active sulfa drugs are produced by substituting N4 position (Fig 25.2).
By substitution at N1 and N4 positions about 5000 compounds are synthesized. Among them 30 are of clinical significance. Sulfanilamide and its derivatives are popularly known as sulfonamide or sulfa drug.
Structure Activity Relations:
(i) Free para amino group is essential for antibacterial activity.
(ii) Substitution of heterocyclic aromatic components at N4 position produces more potent sulfa drugs.
(iii) Any substitution in benzene ring causes loss of activity.
(iv) So2 NH2 group is not essential as such however, sulfur atom is directly linked with benzene ring.
(v) The more negative So2 group at N1 exhibits greater antibacterial activity.
(vi) Substitutions made in the amide NH2 (N1) have variable antibacterial activity.
(vii) The para NH2 group (N4) can be replaced or substituted by such chemical groups that can be converted into free NH2 group in the body.
Classification of Sulfonamides:
1. Based on Chemical Structure:
(i) Both N1 and N4 substituted sulfonamides—
Succinyl sulfathiazole, Phthalyl sulfathiazole
(ii) N1 substituted sulfonamides—
Sulfapyridine (M & B 693), sulfathiazole (M & B 760).
Sulfadimidine (sulfamethazine), sulfamerazine, sulfaphenazole (Orisul), sulfamethoxazole (Sulfuno), Sulfadimethoxine (Madribon), sulfacetamide, Sulfaquinoxaline, sulfaethoxypyridazine
Sulfamethoxypridazine
Sulfasomidine, sulfisoxazole (sulfafurazole)
Silversulfadiazine, sulfamylon (Mafenide)
Sulfasimazole, sulfaguanidine etc.
2. Based on site of action:
(i) Gut acting sulfonamides: Succinylsulfathiazole, phthalylsulfathiazole and sulfaguanidine
(ii) Systemic sulfonamides – N1 substituted sulfonamides (except sulfaguanidine and sulfonamides used topically).
(iii) Renal sulfonamides – sulfisoxazole (sulfafurazole) sulfasomidine
(iv) Topical sulfonamides: Sulfamylon, silversulfadiazine
(v) Ophthalmic sulfonamide: sodiumsulfacetamide (Locula)
3. Based on duration of action:
(i) Long acting – sulfamethazine (Ruminant)
sulfamerazine (Dog), sulfamethoxypridazine, sulfaethoxypridazine
(ii) Short acting-sulfathiazole, sulfasomidine
(iii) Intermediate acting-sulfasimazole and sulfamethoxazole
Antimicrobial Spectrum:
Sulfonamides are broad spectrum antimicrobial agents effective against bacteria, chlamydia, toxoplasma and coccidia. Mycobacteria, mycoplasma rickettsias, pseudomonas and spirochaetes are, however, resistant. They are primarily bacteriostatic but in very high concentration, especially in urinary tract, may be bactericidal.
Sulfonamide therapy should be initiated in rapidly multiplying stage of bacteria i.e. in acute disease conditions. They are less effective in chronic diseases. The sulfonamide therapy is continued till complete removal of infection.
If therapy is discontinued before the complete elimination of microorganisms, more resistant strains of bacteria can emerge. The therapeutic blood concentrations of sulfonamide ranges between 5 to 15 mg%.
Emergence of drug resistant bacteria is frequent. Resistance may develop due to enzyme adaptation, alternate enzyme pathway, chromosomal mutation or plasmid mediation (R-factor). Cross resistance is commonly observed.
While evaluating the potency of the drug, poor correlation is recorded between in vitro and in vivo tests. Higher concentration is required for successful therapy as compared to in vitro results. A MIC of 10-40 µg/ml is indicative of bacterial sensitivity for short acting systemic sulfonamides. MIC of 100 µg/ml indicates bacterial resistance.
Emergence of resistant strain of bacteria can be prevented by:
(i) Avoidance of indiscriminate use of sulfonamide
(ii) Initiation of therapy in acute state of diseases and
(iii) Maintenance of proper bacteriostatic blood level (5-15 mg%) of sulfonamides.
Course of Sulfonamide Therapy:
Too short sulfonamide therapy is generally avoided due to failure of cure. In usual circumstances sulfonamide therapy is recommended for 7 days if a desired response is obtained within 3 days. In no case, sulfonamide therapy should be discontinued before 3 days. Initial doses of sulfonamides are higher for establishing the therapeutic blood concentration and subsequent doses are smaller.
Mode of Action:
Sulfonamides compete with paraaminobenzoic acid (PABA) for the catalytic site of the enzyme dihydropteroate synthatase (Woods, 1940) thereby inhibiting conversion of PABA to folic acid in bacteria. This action of sulfonamide is selective over bacteria without interfering animal cells.
Antibacterial action can however, be reversed by removal of sulfonamide or addition of PABA. Bacterial growth is inhibited by bacteriostatic action. Inhibited bacteria can be eliminated by host defence system preferably by phagocytosis.
Drug Interactions:
Sulfonamides should not be given with procaine penicillin as this combination is antagonistic. Sulfonamides combined with pyrimethamine are the regimen of choice for toxoplasmosis. Ca++ and antacids taken with sulfonamides can inhibit gastrointestinal absorption of sulfonamides. Urine acidification enhances the risk of crystal Luria. Combination of sulfa drug with trimethoprim has synergistic effect.
Pharmacokinetic Properties:
Sulfonamides exist in non-ionized form in biological fluids. They are rapidly absorbed from GI tract and distributed to all tissues and fluids. Distribution of the drug, however, depends upon ionization of drug and barriers to diffusion vascularity of tissues. Drug distribution occurs by passive diffusion.
Metabolism of drug affects its antibacterial toxic activity. Sulfonamides are metabolized in the body by acetylation, oxidation, conjugation with sulphate or glucuronic acid and cleavage of heterocyclic ring. Acetylated, hydroxylated and conjugated sulfonamides have less antibacterial activity. Acetylated drugs cause renal damage because of their insolubility.
Blood concentrations between 5 to 15 mg% is considered safe and efficacious. Acetylation does not take place in dogs. However, toxicity in dog is less because the drug is metabolised by glucuronide conjugation and glucuronide conjugates are soluble. But in cow the high toxicity is due to acetylation. Sulfonamides having longer plasma disappearance half-lives are generally preferred to those having shorter half-lives.
Sulfonamides are excreted as such or as metabolic products. They are eliminated in urine, faeces, bile, milk and sweat. Urine is the main source of their excretion. The mammary gland epithelium is permeable to the non-ionized form of sulfonamides (Rasmussen, 1958)
Routes of Administration:
Oral route-is common route. The drug is available as tablet, bolus, solution and water soluble powder. In ruminants (cattle, sheep) sulfonamides administered orally has depressing and adverse effect on rumen microorganisms.
Intravenous route (IV):
It is generally used for treatment of acute infection. IV administration is followed by oral dose for maintenance therapy. Drug is given slowly by this route.
Intramuscular (IM) or subcutaneous (SC) route:
Specific preparations buffered to a neutral pH may be administered.
Intra-peritoneal route (IP):
It is not common route. Alkaline preparations should not be injected IP.
Intrauterine route:
Drug combined with urea is prescribed for uterine infections.
Topical use:
This route is not suitable for wound treatment due to tissue debris interference with drug. Solution and ointment of sulfacetamide are generally indicated in eye for conjunctival infections.
Adverse Reactions:
A wide variety of side effects are produced. This may be allergic or toxic. Nephrotoxicity is common side effect. Renal damage is characterised by crystalluria, haematuria and obstruction of renal tubules. During therapy water should be readily available to animals to maintain adequate hydration to avoid occurrence of crystalluria.
Aciduria increases the risk of crystalluria hence, sodium bicarbonate is given to make the urine alkaline. In alkaline urine sulfonamides are dissolved and chances of crystal urea reduced. Use of triple sulfonamides (sulfonamide mixture) decreases concentration of each sulfonamide in urine and thus, minimizes the chance of crystal formation.
Anorexia, depression, haematuria, renal colic, frequent urination and rise in BUN level are signs of crystalluria. In case of such symptoms observed, drug is discontinued and fluid therapy is indicated. Increased salivation, vomiting, diarrhoea, hyperpnea, excitement, muscular weakness, ataxia, rigidity of limbs are recorded in dogs when administered @1g/kg. In calf administered IV @ 140 mg/kg weakness, ataxia and collapse occur.
When the drug is continued for longer period, hematopoietic system is de-arranged resulting into thrombocytopenia and leukopenia and liver function is disturbed leading to jaundice. Neuritis and Jaundice are observed in cattle. Cyanosis develops in dogs. Decreased egg production, weight reduction, production of rough thin shelled eggs, liver and kidney lesions, agranulocytosis and anaemia are observed in poultry.
Common Veterinary Drugs, their Doses, Routes and Schedule:
A. Systemic Infections:
(i) Sulfathiazole sodium:
It is given orally or iv.
(ii) Sulfamethazine:
It is pyrimidine sulfonamide generally prescribed orally and parenterally. It is available in oral bolus, IV solution and powder form. It has long half-life and readily absorbed from G.I.tract. IV priming dose is 100mg/kg which is followed by maintenance dose of 50 mg/kg orally at 12 hour intervals.
(iii) Sulfamerazine:
It is mono-methylated pyrimidine sulfonamide used in combination parenterally. It has limited use in veterinary practice.
(iv) Sulfadiazine:
It is also pyrimidine sulfonamide available in combination with trimethoprim for use in dogs.
(v) Sulfabromomethazine sodium (sulfabrom):
It is brominated sulfamethazine. A single oral dose @ 132-198 mg/kg is generally indicated in cattle for therapy of calf diphtheria, pneumonia, metritis, footrot and septic mastitis. In advanced pregnancy drug is contraindicated.
(vi) Sulfaethoxypyridazine:
It is administered orally or IV.
(vii) Sulfadimethoxine:
It is used in form of tablets, bolus or IV injection in companion animals. Its powder form is meant for medication of drinking water. In horses, dogs and cats priming dose is injected IV @50 mg/kg followed by 25 mg/kg at 12 hour intervals.
(viii) Sulfamethoxypyridazine (Midicel):
It is indicated in dog and cats.
(ix) Sulfachlorpyridazine:
It is administered to chickens as solutions or in drinking water.
(x) Sulfisoxazole:
It is given orally in dogs and cats for urinary tract infections @50 mg/kg 8 hourly.
B. Topical Uses:
Sulfacetamide sodium is available as 30% solution or 10% ointment for ophthalmic infections. Mafenide (Sulfamylon) is indicated in burn wounds as cream daily for two times.
C. Gastrointestinal tract infections:
Succinyl sulfathiazole, phthalyl sulfathiazole and sulfa-guanidine are gut active sulfa drugs. They are prescribed in enteric infections of domestic animals.
Oral dose schedule and dosing intervals of few common sulfa drugs are shown in Table 25.4
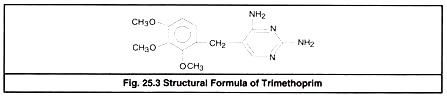
Combinations of Sulfonamides with Trimethoprim or Ormetoprim:
Trimethoprim or ormetoprim are synthetic folic acid antagonists derived from di-amino-pyrimidine (DAP). Individually DAP derivatives and sulfa drugs are bacteriostatic but when combined, their action becomes bactericidal and synergistic. Trimethoprim (Fig 25.3) is widely used in combination with sulfonamides.
In Veterinary preparations, trimethoprim is combined with sulfamethoxazole or sulfadiazine or sulfadoxine in 1: 5 ratio. Trimethoprim is lipid soluble and about 60% bound to plasma proteins. The drug distributes readily by non ionic diffusion attaining effective concentration in most tissues and fluids. Hepatic metabolism (oxidation) is the main process of elimination.
Ormetoprim is closely related to trimethoprim. It is di-amino-benzyl pyrimidine.
Antimicrobial Activity:
Combination has broad spectrum activity effective against aerobic and anaerobic Gram positive and Gram negative bacteria. Enterobacteria, nocardia, chlamydia and protozoa i.e. malarial parasite are sensitive. Combination is also active against some other organisms unaffected by individual components.
Rickettsia, Leptospira, Mycoplasma and Ps. aeruginosa are however resistant. Synergy is observed when organism is sensitive to both components. In some case organisms resistant to one drug are found sensitive to the combination. In synergy combined action is higher to that of summated activity individual components. Generally 10 fold increase in activity of trimethoprim and a 100 fold increase in action of the sulfa drug are recorded.
Resistance:
Bacterial resistance may occur against individual component. Resistance to DAP is usually due to ‘R’ factor plasmid or mutation leading to the synthesis of resistant reductase enzyme. Combination causes resistance through multiple resistant R factors. This has been demonstrated in S. typhimurium and entero-toxigenic E. coli isolated from animals.
Mechanism of Action:
DAP Interferes with the synthesis of tetrahydrofolic acid from dihydrofolate by combining with the enzyme dihydrofolate reductase. Combination inhibits sequential steps in folic acid synthesis i.e. inhibition of synthesis of dihydrofolic acid by sulfa drug followed by inhibition of its reduction by DAP. Purine and pyrimidines are not produced and hence bacterial DNA synthesis is stopped.
Drug Interaction and Adverse Reactions:
The combined preparation can be safely used simultaneously with ampicillin for wide antimicrobial coverage. Trimethoprim sulfonamide combination is highly safe. Blackwell et al. (1989) reported goitrogenic effect in swine resulting to stillborn and weak piglets produced from sows fed with sulfadimelhoxine and ormetoprim in late pregnancy. Prolonged use of combined drug may cause bilateral keratoconjunctivitis in dogs.
Dosage and Administration:
Combined drug therapy is indicated in:
(1) Urinary tract infections
(2) Bacterial prostatitis
(3) Enteric infections due to E. coli, Salmonella spp.
(4) Treatment of brucellosis
(5) Nocardia infections and
(6) Acute upper and lower respiratory tract infections.
In animals usual does is 24-30 mg/kg iv or im at 12 hour intervals. In dogs and cats it is given orally in tablet form. Initial dose is followed by half dose at 12 hour intervals for 2- 3 days after recovery. In horse usual dose is 30 mg/kg twice daily orally (Bertone et al. 1988). In ruminants, the drug is administered parenterally.
In cattle drug is indicated in salmonellosis, diarrhoea, pneumonia, foot rot and urinary tract infection. In acute mastitis IV injection is indicated @ 48-50 mg/kg every 12 hours. The drug is prescribed in swine for treatment of colibacillosis, salmonellosis, and pneumonia. In horses, drug is used for treatment of strangles, urinary tract infections, wounds, abscesses, salmonellosis and protozoal encephalomyelitis (Boy et al, 1990).
It is a drug of choice for urinary tract and ear infections, coccidiosis and prostatic infections of dogs and cats. Trimethoprim and sulfaquinoxaline combination is preferred in poultry for the treatment of E. coli, Pasteurella infection and coccidiosis. The usual dose is 30 mg/kg/day.
Nitrofurans:
Nitrofurans are broad spectrum synthetic compounds. They are primarily used against Gram-positive bacteria. A few Gram-negative bacteria, mycoplasmas, protozoa and fungi are also affected. The 5 nitro group of nitrofurans is concerned with their antimicrobial activity (Fig. 25.4).
Depending upon the concentration used, they may be bacteriostatic or bactericidal. In disk diffusion test, bacteria showing a zone of inhibition equivalent to 25µg/ml are declared susceptible.
The exact mechanism of action of nitrofurans is unknown. They may inhibit an enzymatic oxidative process leading to breakage of bacterial DNA strands and inhibition of DNA synthesis. Resistance develops in bacteria by the use of nitrofurans which is chromosomal and not plasmid mediated.
Nitrofurans are highly toxic when used in high dose for longer time. They are mutagenic and neurotoxic. They may also cause haemorrhagic disthesis, anaemia, anorexia, nausea and vomiting in livestock and cardio toxicity in birds. Their toxicity limits their uses in topical application and treatment of enteric and urinary tract infections.
Most of the nitrofurans are well absorbed when administer orally. They are mainly excreted via kidney in urine. Their metabolism is, however, poorly described. Nitrofurazone and Furazolidone are, however, poorly soluble and hence not absorbed after oral administration.
Nitrofurans and nalidixic acid are not used simultaneously because of antagonistic effect. More than thousand compounds have been developed since the basic structure described in 1930. Only five of them are however found of clinical significance.
Nitrofurazone (Furacin):
It is yellowish powder which is thermo-stable and slightly soluble in water. It is used topically for treatment of wounds and skin, eye, ear and reproductive tract diseases. In birds it is given orally for prevention of coccidiosis. It is given in pig feed @100- 500 ppm or 50-500 G/900 kg or 100 mg/L water for prevention of swine enteritis.
Nitrofurantoin (Furadantin):
It is bitter powder, possessing broad antibacterial action against Gram-positive and Gram-negative organisms. It is absorbed from GI tract without disturbing intestinal bacteria and 40% is excreted unchanged in urine. The concentration attained in the urine is bactericidal.
It is exclusively used for treatment of urinary tract infections. In dog the oral dose is 4 mg/kg three times daily for 5-7 days. It may be given IM @ 3.3 mg/kg twice daily, In man it is given orally @ 50-100 mg four times daily for urinary tract infections.
Furazolidone (Furoxone):
It is also yellowish powder generally used as feed additive. It has the greatest antibacterial activity followed by nitrofurazone and nitrofurantoin. It interferes acetylation of CoA.
It is given for prevention of coccidiosis and bacterial enteric infections in poultry and enteric infections in swine caused by E. coli and Salmonella sp. In horses it is used @4.4 mg/kg three times daily orally for the treatment of foal diarrhoea. As feed additive for swine and poultry it is mixed @ 10-2000 G/900kg feed.
Furaltadone:
It is readily absorbed from the gastro intestinal tract. It is indicated for the treatment of bovine mastitis @500mg/quarter. It is added @13.2 mg/kg in milk replacer for prevention of colibacillosis in calves. In poultry it is effective against fowl typhoid S. typhimurium infection and mycoplasmosis. For treatment it is used as 0.04% in drinking water of chickens.
Nifuroquine:
It has been used for treatment of Mycoplasma bovis mastitis bacterial mastitis and dry cow therapy.