A research paper on acid rain. After reading this you will learn about: 1. Meaning of Acid Rain 2. Sources of Acidic Pollutants 3. Measuring Acidity 4. Effects 5. Control Strategy 6. Legislative Control.
Contents:
- Research Paper on the Meaning of Acid Rain
- Research Paper on the Sources of Acidic Pollutants
- Research Paper on the Measuring Acidity of Acid Rain
- Research Paper on the Effects of Acid Rain
- Research Paper on the Control Strategy of Acid Rain
- Research Paper on the Legislative Control of Acid Rain
Research Paper # 1. Meaning of Acid Rain
:
Acid rain is a widespread term used to describe all forms of acid precipitation (rain, snow, hail and fog). Atmospheric pollutants, particularly oxides of sulphur and nitrogen, can cause precipitation to become more acidic when converted to sulphuric and nitric acids, hence the term acid rain.
Acid deposition, acid rain and acid precipitation all relate to the chemistry of air pollution and moisture in the atmosphere. UK scientists generally use the term acid deposition but all three terms relate to the same issue.
The term acid rain was first used by Robert Angus Smith, a scientist working in Manchester in the 1870s. The problem of acid rain is hence not a new one but the nature of the problem has changed from being a local problem for towns and cities to being an international problem.
In Smith’s time, acid rain fell both in towns and cities whilst today pollutants can be transported thousands of kilometers due to the introduction of tall chimneys dispersing pollutants high into the atmosphere.
Research Paper # 2. Sources of Acidic Pollutants:
Precipitation is naturally acidic because of carbon dioxide in the atmosphere. The burning of fossil fuels (coal, oil and gas) produces sulphur dioxide and nitrogen oxides which can increase the acidity of ram or other precipitation. Sources of sulphur dioxide and oxides of nitrogen may be natural such as volcanoes, oceans, biological decay and forest fires, or may arise from combustion sources.
The increasing demand for electricity and the rise in the number of motor vehicles in recent decades has meant that emissions of acidifying pollutants have increased dramatically from human sources, particularly since the 1950s. Emissions of such pollutants are heavily concentrated in the northern hemisphere, especially in Europe and North America. As a result, precipitation is generally acidic in these countries.
In the 1970s and 1980s, Scandinavian countries began to notice the effects of acid deposition on trees and freshwaters. Much of the pollution causing this damage was identified as being transported from other more polluting countries. Acid rain became an international concern.
Research Paper # 3. Measuring Acidity of Acid Rain:
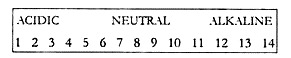
The pH (not PH) scale is used to measure the acidity or alkalinity of an aqueous solution and is determined by the hydrogen ion content (H+). This scale was invented by a Danish scientist called Sorenson in 1909. The pH scale ranges from 0, which is strongly acid, to 14 which is strongly alkaline, the scale point 7 being neutral.
Examples of solutions with differing pH values are as follows:
1. pH 1 car battery acid
2. pH 2 lemon juice
3.pH 3 apple
4. pH 4 beer
5. pH 5-6 natural rain
6. pH 6 milk
7. pH 7 washing up liquid
8. pH 8 sea water
9. pH 10 milk of magnesia
10. pH 12 ammonia
The pH scale is logarithmic rather than linear (Fig. 16.1), and so there is a tenfold increase in acidity with each pH unit, such that rainfall with pH 5 is ten times more acidic than pH 6, rainfall with pH 4 is 100 times more acidic than pH 6 and rainfall with pH 3 is 1000 times more acidic than pH 6.
Rainfall acidity is measured in pH units. The individual pH readings may be converted to hydrogen ions to give a linear rather than a logarithmic representation of acidity. To convert the pH values to hydrogen ions, the following formula applies:
H+ µeql-1 = antilog (6.0 – pH)
where H+ µeql-1 is the hydrogen ion content in micro equivalents per litre (a unit which measures the concentration of hydrogen ions in a litre of water).
For example, the H+ ion content of rainwater with pH 4.54 is:
H+ µeql-1 = antilog (6.0 – 4.54) = 29
The hydrogen ion content of a number of pH values are given in the following table and are illustrated on Fig. 16.1.
‘Normal’ or ‘unpolluted’ rainfall has a pH of 5.6 (3 µeql-1 H+). This is slightly acidic due to the presence of carbon dioxide in die atmosphere winch forms weak carbonic acid in water. The rainwater pH for the example given above (pH 4.54) is almost 10 times more acidic than ‘unpolluted’ ram.
Research Paper # 4. Effects of Acid Rain:
Acid rain produces complex problems and their effects are:
(a) Effect on Fishes:
Due to acid rain many water bodies in Germany and other European countries have a pH of less than 5. This acidity level is considered to be lethal for many fish species. Acidic water can also leach aluminium from the soil and carry this dissolved aluminium to lakes and streams.
In alkaline or new-neutral lakes aluminium concentrations present in rocks, soil and sediments are very low. As the pH decreases, the previously insoluble aluminium goes into solution. This dissolved aluminium clogs the gills of fish and deprives them of oxygen.
(b) Effect on Lake Ecosystem:
Thousand of lakes in USA, Canada, and Norway etc. have become unproductive due to acidity. It has low levels of phytoplankton. Snails, clams and other animals with shells (calcium carbonate) gets readily dissolved by acid water (as given below) and are among the first animals to die and are followed by fishes:
CaCO3 + H2SO4 → CaSO4 + CO2 + H2O
Some insects like dragon fly larvae, water boatman etc. replace fishes at the top of the food chain in acidic lakes. Aquatic plants (broad-leafed pondweeds etc.) die and the lake becomes covered with moss mats. Bacteria and other microscopic animals are also reduced.
(c) Effect on Terrestrial Ecosystem:
In terrestrial plants acid rain causes:
1. Reduced rate of photosynthesis and growth.
2. becomes sensitive to drought and diseases.
3. Retarded growth in a variety of crops like beans, radish, spinach etc.
4. Activity of nitrogen fixing bacteria are severely affected which ultimately hampers the nitrogen cycle.
5. Trees like spruce, pine ashes, birch etc. appear to be highly vulnerable to acid deposition (in Germany nearly 8 percent of forest trees died due to acid rain).
(d) Effect on Materials:
Acid rain causes extensive damage to paints steel, plastic, building and sculptured materials of marble, limestone, slate, mortar etc. The Taj Mahal of India faces a grave situation due to acid rain.
(e) Effect on Human Health:
Atmospheric acidity causes chronic respiratory problems. Heavy metals such as copper, cadmium, zinc, mercury etc., are liberated from soil and bedrock by acid rain which eventually reach the human body via plants and animals in the food chair or through drinking water supplies.
Research Paper # 5. Control Strategy of Acid Rain:
There are two major control strategies which are adopted viz., vehicular emission control and industrial emission control. Most of these strategies are already applied in Europe.
1. Vehicle Emission Control:
In 2000 there were 24.8 million passenger cars and light goods vehicles licensed in the UK. In 1950 there were 2.4 million, over a ten fold increase in licensed UK cars and light goods vehicles in just 50 years. World car populations have increased at a similar rate from around 50 million in 1950 to an estimated 630 million in the early 1990s and predicted escalation to 1,000 million by the 2020s.
UK consumption of energy during 2000 accounted for 160.1 million tonnes of oil equivalent. Motor vehicles accounted for over a third of this, and are consequently a major contributor to gaseous air pollution, in particular from nitrogen oxides, hydrocarbons and carbon monoxide. In 1999, road transport contributed considerable air pollutants (Table 16.3).
A motor vehicle produces air pollutants when fuel is burnt to give mechanical power. In a totally efficient combustion process, hydrocarbons and oxygen will react to form carbon dioxide and water. However, the combustion process is never perfect; some of the hydrocarbon fuel is only partially burnt forming carbon monoxide and water, whilst some of the hydrocarbons are not combusted at all.
These can, and often are, emitted from the exhaust as unburned hydrocarbons. During the combustion process the temperature can reach 250°C. At these temperatures nitrogen and oxygen from the air in the combustion chamber react to form nitrogen oxides.
Vehicle pollution can be significantly reduced by fitting a catalytic converter to the exhaust system. This is a relatively low cost method of pollution control (around £350) which has little effect on vehicle performance and fuel consumption. All new cars sold in Britain from January 1993 onwards have catalytic converters but most cars bought before 1993 will not have one fitted.
The most widely used catalytic converter consists of a cylindrical ceramic body with a honeycomb structure, chemically treated and coated with platinum group metals. The honeycomb structure enables a high surface area (equivalent to three football pitches) to be incorporated within a relatively small space.
This is critical to the durability and reliability of performance. The catalyst is usually incorporated into the car exhaust system (Fig. 16.2).
There are three basic types of catalyst, an oxidation catalyst which controls the emission of hydrocarbons and carbon monoxide by oxidising the pollutants to water and carbon dioxide, a three way catalyst which provides efficient removal of nitrogen oxides, carbon monoxide and hydrocarbons, and a diesel catalyst which controls the emissions of hydrocarbons (the characteristic diesel smell) and carbon monoxide.
The catalyst will also substantially reduce smoke emissions.
The United States of America and Japan first introduced tough standards for controlling emissions from motor vehicles in the early 1970s. The US standards can only be met by the use of catalytic converters. Many European countries including Switzerland, Austria, Sweden and Norway also adopted US standards during the 1980s.
In 1990, a European Community Directive stated that all petrol engine cars sold in EC countries from January 1993 must be fitted with a three way catalytic converter. Europe has been slow to act on the link between vehicle emissions and acid rain and air quality.
Directly and indirectly vehicle emissions make a significant contribution to the cause of acid deposition, the formation of photochemical smog and are a potential risk to human health.
The major gases leading to the formation of acid .deposition arc sulphur dioxide (SO2) and nitrogen oxides NOX. Vehicles do not produce much sulphur dioxide as petroleum contains very little sulphur. In 1999, vehicles contributed only 1% of the UK SO2 emissions whilst power stations being the major source of the total UK SO2 emissions contributed 65%.
Nitrogen oxides from vehicles are however, a major contributor to acid deposition in the UK.
In 1999, road transport accounted for 44% of NOx emissions. The atmospheric chemistry of nitrogen oxides is complex but generally speaking, the nitrogen oxides arising from motor vehicles may either be oxidised to nitric acid or react with hydrocarbons to form ozone.
Photochemical smog, sometimes called summertime smog is the result of the sunlight-stimulated reacuon between hydrocarbons and nitrogen oxides leading to the formation of low level atmospheric ozone and other chemical oxidants.
Such smog’s and hazes are typical of warmer cities, a particularly well known example being Los Angeles. However, many instances of photochemical smog’s have occurred in the UK in recent years which may be due to the growth of vehicle ownership.
Winter time smog’s also occur when atmospheric pollutants, mainly those found in car exhausts, arc trapped at ground level by a layer of colder air above towns and cities. During high pressure conditions warm air rises during the daytime but if this is followed by a cloudless night, ground level temperatures fall and cold air rises but is trapped by warmer air above. The cold air forms a lid if conditions are calm, trapping pollutants from vehicles and other sources.
Health risks associated with car exhaust emissions are usually connected with carbon monoxide which reduces the oxygen carrying capacity of the blood. However, nitrogen oxides can cause breathing problems at high concentrations and therefore sufferers from emphysema and chronic bronchitis are likely to be most at risk.
Some of the hydrocarbons found in vehicle exhaust fumes are known to be carcinogenic. Indirect effects of vehicle emissions on health may arise when air pollution episodes occur such as summertime or wintertime smog’s. At such times, those suffering from heart or lung diseases may find that their symptoms worsen.
The increase in use of catalytic converters on vehicles should in the long term help to reduce emissions of pollutants from vehicles although the continued growth in car ownership may impede this improvement.
2. Industrial Emission Control:
Acidic emissions of sulphur dioxide (SO2) and nitrogen oxides (NOX) arise from many industrial sources as a result of combustion processes. In the UK in 1999, power stations contributed 65% of all SO2 emitted in the UK. Other industries were responsible for 21%.
Industries also emit nitrogen oxides which can also cause rainfall to become more acidic. While road transport is the major source of NOx in the UK (44% in 1999), power stations accounted for 21% and other industries 13% in 1999. There are many technologies which can be used in industry to reduce the emissions of pollutants to the atmosphere and these can be applied before, during or after combustion.
3. Pre-Combustion and During Combustion Technology:
Examples of pre-combustion technology include coal scrubbing and oil desulphurization. Another removal process is to change the design of the boiler and to install pressurised fluidized bed combusters (FBC) which removes sulphur from coal during coal during combustion.
Another process which removes sulphur dioxide from coal during combustion is the Integrated Gasification Combined Cycle. Coal is gasified under pressure with a mixture of air and steam which results in the formation of gas which can then be burned to produce electricity.
4. Post-Combustion Technology:
One of the post-combustion controls is Flue Gas Desulphurization (FGD). In FGD processes, waste gases are scrubbed with a chemical absorbent such as limestone to remove sulphur dioxide. There are many different FGD processes, the main ones being the limestone-gypsum process and the Wellman-Lord regenerative process.
The limestone-gypsum FGD is installed at Drax in Yorkshire, the largest UK power station. This technology involves mixing limestone and water with the flue gases to produce a slurry which absorbs the sulphur dioxide.
The slurry is then oxidised to calcium sulphate (gypsum) which can then be used in the building trade. However, whilst the benefits of FGD are known, the costs of installing FGD are very high. To install a 2GW power station with FGD costs around £300 million.
5. Fuel Efficiency:
Some fuels are naturally less polluting in terms of acidic emissions (e.g., gas), whilst the traditional coal power generation in the UK is more polluting, depending on the amount of sulphur there is in the coal being burnt.
To help reduce atmospheric emissions of SO2 and NOx in the UK, many of the more recent power stations have been built to operate on gas rather than coal. Table 16.4 compares the relative efficiencies and emissions of different fuel types from UK power stations.
The current trend in the UK is a move from gas to coal due to the low levels of sulphur dioxide, nitrogen oxides, dust and carbon dioxide emitted when gas is burned. However, whilst gas is currently plentiful, availability in the long-term is unknown.
Hence, research is being carried out into techniques for reducing sulphur dioxide emissions from coal such as FBC and IGCC. Such techniques are unlikely to be used on a commercial scale until well after the year 2000.
Research Paper # 6. Legislative Control of Acid Rain:
The UK, as a member country of the European Union, is under obligation to comply with the Large Combustion Plant Directive of 1988 which concerns reductions in emissions of SO2 and NOx from plants over 50MW in size. The UK is, under this Directive, required to reduce SO2 by 60% by 2003 and NOx by 30% by 1998 (from 1980 levels).
The UK is also a Party to the Gothenburg Protocol, designed to Abate Acidification, Eutrophication and Ground-level Ozone. The UK is committed to reducing 1990 emissions of sulphur dioxide by 75% by 2010 and nitrogen oxide by 50% over the same period.
In addition, the UK National Air Quality Strategy sets out standards and objectives for reducing key air pollutants to be achieved by the year 2005. To meet the objectives for the acid deposition pollutants (largely SO2 and NOx), industry will have a significant part to play in reducing emissions.
As most of the SO2 emitted in the UK is from industry and power generation, reduction in annual emissions will be necessary from industrial sources. The use of cleaner fuels and the use of control technologies will be required at all new industrial plants if SO2 levels are to continue in a downward trend.
There can be no quick solution to the control of acid rain. However, certain actions can be taken as fundamental requirements, such as
(i) The recognition that acid rain is a serious problem.
(ii) The knowledge that reduction of emissions is the best solution.
(iii) Need for regular monitoring to provide warning about acidification of our environment.
Short term control measures of acid rain can be achieved by the use of lime, so as to provide the lakes with an alkaline pH. Some ecologists have suggested applying lime to entire water-sheds, including forests rather than just to individual lakes. However, such actions require the involvement of a large sum of money.