This article throws light upon the three types of air control devices.
They are: (1) Control Devices for Particulate Pollutants (2) Control Devices for Gaseous Pollutants and (3) Control Devices for Volatile Organic Compounds (VOCs).
The air pollution load particularly from industries can be reduced by several measures- replacement of burning fuel by electricity or solar energy, improvement in fuel burning process, dispersion and dilution of pollutants, and reduction at source by using control equipment. These measures, however, are useful only to a limited extent.
In the nature itself, there are certain devices, commonly referred to as atmospheric self-cleansing processes for the removal of air pollutants. These natural processes are very slow and limited, and cannot cope up with the present increased demands of pollution control.
However, the artificially devised pollution control measures are based on the same principles of atmosphere self- cleansing processes. These principles include dispersion, gravitational settling, flocculation, absorption and rain out.
There are two categories of air control devices—devices to control particulate pollutants, and to control gaseous pollutants.
1. Control Devices for Particulate Pollutants:
In general, greater emphasis is given to control particulate pollutants, may be because they are visible. It may however, be noted that in terms of volume of air pollution, gaseous pollutant contribution is much higher.
The important devices/equipment used to control particulate pollutants are listed:
i. Gravity settling chambers
ii. Cyclone collectors
iii. Dynamic precipitators
iii. Spray towers
iv. Electrostatic precipitators
vi. Fabric filters.
Gravity settling chambers:
Settling chambers are the oldest and very simple type of equipment used for collection of solid particles (Fig. 55.1A). As the air is passed through the chambers at a low velocity, the dust particles (size 40-100µm diameter) settle by gravity and the clean air comes out. Although this technique is simple, inexpensive with low cost of maintenance, it is not possible to settle smaller size dust particles.
Cyclone collectors:
Cyclone collectors also operate on the principle of gravity settlement. The equipment mainly consists of a vertically placed cylinder with an inverted cone attached to its base (Fig. 55.1B). As the air enters the cylinder, it takes a helical path downwards.
Due to rapid spiraling movement of the air, the particles are thrown towards the walls by centrifugal force. These dust particles settle down in the hopper at the bottom, while the dust free air passes through a pipe and comes out. The cyclone collectors are simple, low cost and easy for maintenance. They are ideal for separation of dust particles with sizes 15-50µm. Cyclone collectors cannot settle particles less than 10µm size.
Dynamic precipitators:
Dynamic precipitators work on the principle of centrifugal force, generated by rotating blades (Fig. 55.1C). The dust particles of the air are concentrated on the rotating blades from where they are collected in a concentrated stream. By employing this equipment, dust particles of 5-20 nm size can be separated. However, dynamic precipitators are not suitable for sticky or fibrous dust particles, as they stick to the blades.
Spray towers:
In spray towers, the dust particles (10µm in size) are made to settle down by spraying water (Fig. 55.1D). This technique is particularly useful for separating a heavy load of particles.
Electrostatic precipitators:
Electrostatic precipitators (ESPs) are very efficient and versatile. They work on the basis of electrostatic attraction. An ESP consists of a thick cylinder fitted with an inlet at bottom and an outlet at the top (Fig. 55.1E). An electrode (more than one electrode are commonly used) fitted at the centre of the cylinder is connected to a high voltage cable.
As the dust laden air passes through ESP, larger sized particles settle down due to gravity. The smaller charged particles are attached to the appositively charged electrodes which gradually fall down to the bottom. The dust free air comes out.
Electrostatic precipitators are said to be dry precipitators if the dust particles can be removed by scrapping. In case of wet electrostatic precipitators, the particles are removed by using water or any other fluid. Wet precipitators are more efficient; however, dry precipitators are preferably used for practical reasons.
Fabric filters:
Fabric filters (Fig. 55.1F) are the most efficient and can separate particles with sizes less than 0.5µm in diameter. As the air or gas is passed over fabric like mats of wool, celluloses etc., the dust is trapped while the gas passes out. Collection of dust on the fabric filter results in the formation of a dust layer (commonly referred to as filter cake). This in turn serves as a more efficient dust collector and helps to capture even fine dust particles. The fabric filters have to be frequently cleaned. Several models of fabric filters have been developed for commercial applications.
2. Control Devices for Gaseous Pollutants:
The principle gas pollutants are SO2, NO2, N2O, CO, hydrocarbons, and organic and inorganic acid gases. The control devices used for gaseous pollutants are based on the principles of adsorption, absorption, condensation and combustion. The important aspects of selected devices for controlling gaseous pollutants are briefly described.
Adsorption:
When a stream of gas is passed through a porous solid material (absorbent), it can attract and hold the gas (adsorbate) molecules. If the adsorption is purely a physical phenomenon (held by van der Waal’s forces), it is referred to as physical adsorption. This process is rapid and easily reversible. In chemical adsorption (chemisorption), the pollutant gas molecules form chemical bonds with the adsorbent. Chemisorption is very slow, usually requires the supply of energy and is irreversible.
Pressure and temperature significantly influence chemical adsorption. In the Table 55.6, a selected list of the commonly used adsorbents along with their major applications is given. The equipment used for adsorption, referred to as adsorbers, may be fixed, moving or fluidized beds.
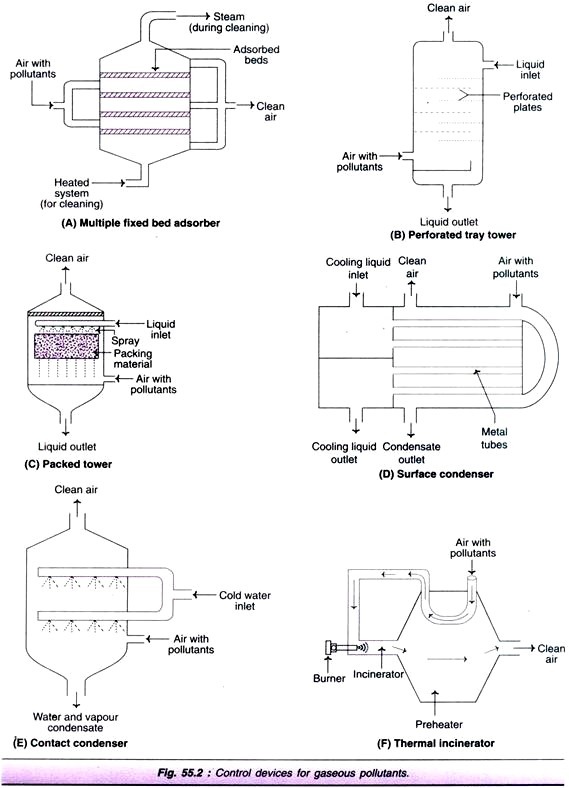
Multiple fixed bed adsorber:
In this, adsorbent is arranged in the form of beds or layers as depicted in Fig. 55.2A. As the adsorption occurs, the adsorbent gets saturated with the adsorbate. For reuse, the gas collected on the adsorbent must be removed. If this is not possible, the adsorbent must be replaced.
Absorption:
The pollutant gas (absorbate), when brought in contact with a solvent or liquid (absorbent), gets adsorbed. The process of absorption may occur due to physical or chemical phenomena. Absorption process is used to control the pollutants SO2, NO2, H2S and hydrocarbons. Ammonia is used as an absorbent for controlling SO2.
Different types of absorbers are used for control of gaseous pollutants. These include spray towers, plate or tray towers and packed towers. The gas absorption units are so designed that there occurs an intimate contact between the gas and the liquid. This will ensure optimal absorption of gas into the liquid.
Spray towers:
They can efficiently remove gaseous pollutants, besides the particulates. These devices are particularly useful for the removal of pollutant gases with high concentrations of particulates.
Plate or tray towers:
These absorbers are designed with horizontal trays or plates so as to provide large liquid-gas interfacial surface (Fig. 55.2B). In the perforated tray tower, the absorbent enters the column from the top, spills and flows in a zigzag fashion. The polluted air enters the column from an inlet at the side of the bottom. As the air passes through the openings of the trays, it gets in close contact with liquid due to repeated exposure. This enables the removal of gaseous as well as particulate pollutants. The clean gas emerges at the top.
Packed towers:
In this case, the liquid (absorbent) is sprayed over a packing material (e.g. all rings, Berl saddles) with large surface to volume ratio (Fig. 55.2C). The air with gaseous pollutants enters from the side of the bottom of the tower and the clean air comes out from the top.
Condensation:
When the temperature of a gaseous compound is reduced, its condensation occurs. There are two devices commonly used to control gaseous pollutants, based on the principle of condensation.
Surface condenser:
In this device, the cooling medium is passed through metal tubes over which the gas with pollutants is passed (Fig. 55.2D). The vapour condenses on the surface of the tubes. This gets collected as a film of liquid which can be drained off.
Contact condenser:
In contact condenser, the cooling medium and the gaseous pollutants are made to have a direct contact with each other (Fig. 55.2E). As the vapors get cooled, they condense and are easily removed along with the water, and the clean gas comes out from the top of the condenser.
Combustion:
It is a fact that combustion or incineration is a major source of air pollution. And surprisingly, the same combustion processes effectively serve to control air pollutants. This is carried out by converting certain pollutants (e.g., hydrocarbons, carbon monoxide) to carbon dioxide and water. For efficient oxidation during combustion, supply of oxygen, optimal temperature and time are necessary. There are different types of combustion processes depending on the pollutant to be oxidized. The most important ones are direct-flame combustion, thermal combustion and catalytic combustion.
Direct-flame combustion:
In this device, the waste gases are directly burnt with or without the addition of auxiliary fuels. Direct-flame combustions are most often used in petrochemical plants and refineries.
Thermal combustion:
This process usually becomes necessary when the gaseous pollutant concentration is too low, and it is difficult to carry out direct-flame combustion. Thermal combustion is carried out by a thermal incinerator or after burner (Fig. 55.2F). An heat exchanger preheats the air/gas with gaseous pollutants. This preheated gas is passed over to the incineration equipped with a burner.
The temperature of the incinerator may be in the range 500-1000°C, and this actually depends on the nature of the pollutant to be oxidized. Combustion with thermal incinerators has to be carefully carried out so that the oxidation process is efficient and complete. Incomplete combustion may produce more pollutants e.g. carbon monoxide.
Catalytic combustion:
This is required when the gaseous pollutants are too low in concentration. By employing a catalyst, the combustion can be made faster and complete, and the catalyst can be used again and again. Catalytic combustion is used for the removal of NO2 (in the tail gas) from the nitric acid plants.
Here platinum catalyst is used. Carbon monoxide can be removed by using copper (Cu2+) catalyst. The major limitation of catalytic combustion is the high cost of the catalyst and maintenance of the catalytic combustion.
3. Control Devices for Volatile Organic Compounds (VOCs):
There are several volatile organic compounds, arising from industrial and domestic activities that cause air pollution. Good examples of VOCs are alcohols, ketones, organic acids, phenols and organic solvents. The conventional methods (e.g. combustion, adsorption) of treatment VOCs require large quantities of energy, besides creating secondary pollution. Biodegradation by microorganisms is a preferred method for controlling air pollution of VOCs.
The efficiency of biodegradation depends on the individual compounds and based on this, VOCs are broadly categorized as follows:
i. Rapidly degraded VOCs—alcohols, ketones, aldehydes, organic acids.
ii. Slowly degraded VOCs—hydrocarbons, phenols, organic solvents (chloromethane).
iii. Very slowly degraded VOCs—polyaromatic hydrocarbons, polyhalogenated hydrocarbons.
There are mainly two types of reactor designs for the biodegradation of VOCs — bio-filters and bio-scrubbers (Fig. 55.3).
Bio-filters:
A bio-filter is composed of a porous medium (of compost, wood chips) that supports the microorganisms forming a film. The polluted air is first passed through a humidifier and then through the bio-filter. A great majority of VOCs in the air (approximately 90%) get biodegraded, and clean air comes out of the bio-filter (Fig. 55.3A).
Advantages of bio-filters:
They are simple and cost-effective. Certain poorly soluble pollutants (hydrocarbons) can be easily removed by this approach. Bio-filters can be successfully used for the biodegradation of several xenobiotic e.g. chloromethane.
Disadvantages of bio-filters:
It is rather difficult to control process conditions (pH, temperature). When compost is used as support material, it generates unpleasant odours. Bio-filters require large areas.
Bio-scrubbers:
Bio-scrubber is an improvement over bio-filter (Fig. 55.3B). When the polluted air is passed through a liquid stream in a spray chamber, the pollutant (VOC) gets transferred to the liquid. The sprayed liquid contains a suspension of microorganisms which cycles between the spray chamber and waste water treatment unit (activated sludge unit). The microbial biodegradation of VOC occurs in the waste water treatment unit. The clean air comes out, after recycle, from the spray chamber. Besides a continuous nutrient supply, it is essential to maintain the pH in the activated sludge unit.
Bio-trickle filters:
Bio-trickle filter is a recent development for more efficient biodegradation of VOCs. It works on the principles of adsorption, followed by biodegradation. The pollutant gases are adsorbed on a solid surface (e.g. activated carbon) and then subjected to biodegradation by a stream of medium containing the desired microorganisms. As of now, bio-trickle filters are at an experimental stage, and soon they may be available for use.