The theories are: 1. The Chemical Coupling Theory 2. The Conformational Coupling Theory 3. The Chemiosmotic Theory 4. Binding-Change Mechanism of ATP Synthesis Theory.
Mechanism of Energy Transduction: Theory # 1.
The Chemical Coupling Theory:
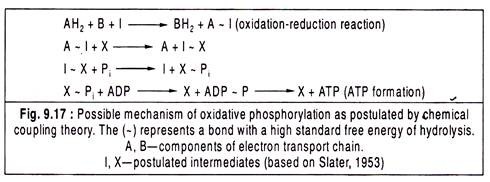
Slater (1953) originally suggested that during the oxidation of a component of electron transport chain, the energy released is trapped by the formation of a bond (written as ~) between that component and some other molecule that is not the part of the chain.
Mahler and Cordes (1971) developed a model for respiratory chain-linked phosphorylation which is analogous to the reactions of oxidative phosphorylation at the substrate level.
Two examples were cited:
(i) The oxidation of glyceraldehyde-3-P to 3-phosphoglycerate by the enzymes glyceraldehyde-3-P dehydrogenase and 3-phosphoglycerate kinase and
(ii) The oxidation of succinyl CoA to succinate by succinate thiokinase. In reaction (i), Pi is directly incorporated into one of the products of the redox reaction (i.e., 1,3-bisphosphoglycerate) and then transferred to ADP, while in reaction (ii), a reactive intermediate (i.e., succinyl CoA) without P. is generated which is subsequently transformed into ATP (Fig. 9.6).
The following reaction schemes were proposed by Slater (1953) to explain the chemical coupling theory:
Mechanism of Energy Transduction: Theory # 2.
The Conformational Coupling Theory:
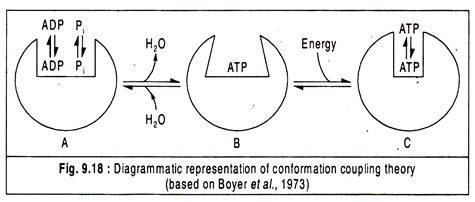
The conformational coupling theory is based on the concept that an enzyme undergoes a conformational change when a product is released. The enzyme adenosine triphosphates (ATPase) is responsible for catalysing ATP synthesis from ADP and Pi which is quite reverse of the normal ATPase reaction catalysing the hydrolysis of ATP into ADP and Pi.
This theory suggests that energy is required for a conformational change in the ATPase enzyme which leads to a change in its affinity for its substrate (ADP and Pi) or product (ATP) causing substrate binding and product release (Fig. 9.18).
According to Boyer et al. (1977), the sequence of events starts with a loose binding of ADP and Pi on ATPase, then a conformational change in the ATPase brought about by energy input resulting in a shift from a loose to a tight binding active site.
ATP is formed but its release does not occur until a second conformational change alters the active site of the ATPase to again become a loose binding site so that the newly synthesized ATP can be liberated.
Mechanism of Energy Transduction: Theory # 3.
The Chemiosmotic Theory:
The chemical coupling theory suffers from the drawback in that it fails to consider the role of membranes in which energy transduction takes place. The importance of the membrane was emphasized by Peter Mitchell who developed the chemiosmotic theory based on the concept of protocity, i.e., the transport of protons (H+ ions) which is analogous to electricity, i.e., the transmission of electrons (Fig. 9.19).
According to Mitchell’s theory, the phosphorylation process (i.e., ATP synthesis) is separated from the oxidation-reduction reactions of the electrons of the electron transport chain. Phosphorylation is thought to occur on the membrane associated with a reversible proton-pumping ATP synthase and this process may occur in the complete absence of electron transport.
The energy liberated during the passage of electrons through the electron transport chain is able to develop a gradient of protons or pH gradient across the inner mitochondrial membrane which is impermeable to protons.
When this gradient or proton motive force develops to sufficient amount, the pressure of the gradient on the proton trans-locating ATP synthase becomes very high and the discharge of the gradient through the ATP synthase releases the energy required for ATP synthesis.
According to the chemiosmotic theory, the electron transport chain is coupled with the ATP synthesizing complex only through the membrane potential.
The theory states that the free energy of electron transport is conserved by pumping H+ from the mitochondrial matrix to the inter-membrane space to create an electrochemical H+ gradient across the inner mitochondrial membrane. The electrochemical potential of this gradient is utilized to synthesize ATP.
The key observations of the theory are given below:
1.The oxidative phosphorylation requires an intact inner mitochondrial membrane, because a closed compartment is essential for the generation of electrical potential gradient. ATP synthesis coupled to electron transfer does not occur in soluble preparations or in membrane fragments lacking well-defined inside and outside compartments. 
2. The inner mitochondrial membrane is impermeable to ions such as H+, OH–, whose free diffusion would discharge the electrochemical gradient.
3. Electron transport through the respiratory chain results in the transport of protons into the inter-membrane space, thereby creating a measurable electrochemical gradient across the inner mitochondrial membrane. The pH outside is 1.4 units lower than the matrix, and the membrane potential is 0.14V.
The outside becomes positive and the matrix side becomes negative. The total proton motive force that can be developed is 0.22V that corresponds to a free energy of 5.2 kcal per mole of protons.
4. Compounds (ionophores) that increase the permeability of the inner mitochondrial membrane to protons and thereby dissipate the electrochemical gradient allow electron transport to continue but inhibit ATP synthesis.
That means, they “uncouple” electron transport from oxidative phosphorylation. Conversely, if acidity can be imposed outside the inner mitochondrial membrane, it stimulates ATP synthesis without electron transport.
5. Both the respiratory chain and the ATP synthase are vectorially organised in the inner mitochondrial membrane.
Mitchell proposed that the components of the electron transport chain, viz., the electron carriers and hydrogen carriers are arranged in such manner that they transport protons across the mitochondrial membrane.
Oxidation-reduction reactions on the membrane are vectorially arranged so that when one component in the electron transport chain is reduced by accepting electrons from the previous electron carrier, it takes up protons from the inside (i.e., matrix side) of the inner membrane of mitochondria.
When the reduced component in turn undergoes oxidation by the next electron carrier, it releases protons on the outside (i.e., cytosolic side) of the membrane. Since the oxidation and reduction reactions are vectorially placed on opposite sides of the membrane, there will be a net transport of protons across the membrane.
There are three proton-trans-locating sites in the mitochondrion which are mediated by FMN (Complex I), UQ and cytochrome a-a3 (Complex IV), i.e., cytochrome oxidase and these correspond with three coupling sites for ATP synthesis.
The actual phosphorylation process, viz., ATP synthesis takes place on an ATP synthesizing enzyme system which is located on the inner mitochondrial membrane. The enzyme ATP synthase has diverse functions. In the hydrolytic mode of action, the ATP synthase can catalyze the hydrolysis of ATP to ADP and P1. but in the intact system it catalyses the reverse reaction, i.e., the dehydration of ADP and P1 to form ATP and water.
Another function of ATP synthase is that it pumps protons outside during hydrolytic activity, whereas the protons may pass inside through the ATPase during synthetic activity. The enzyme complex meant for ATP synthesis can be more appropriately called ATP synthase or F0F1 ATPase instead of simple ATPase.
Two major components of this enzyme complex are F0 and F1 which may be regarded as coupling factors functioning in the coupling of ATP synthesis to electron transport. F1 (factor 1) component projects into the matrix from the inner membrane which is attached to the base component F0 (o for oligomycin) by a stalk.
F0 is a specific protein factor which when added to the F, results in the inhibition of the ATP synthase activity by oligomycin. Hence F0 is also called oligomycin sensitivity conferring factor or OSCF. There is a channel through F0 that specifically trans-locates protons on both sides.
John Walker deduced the high-resolution structure of the F1 part of ATP synthase enzyme and jointly owned the Nobel Prize with Paul Boyer in 1997 in chemistry. The enzyme has nine subunits of five different types, with the composition α3 β3 γ δ ε.
Each of the β subunits has one catalytic site for ATP synthesis. The F1 portion is a flattened sphere measuring 8 nm high and 10 nm across consisting of alternating α and β subunits arranged like the section of an orange.
The F0 complex that makes the proton pore is composed of three subunits a, b, and c in the proportion a b2c10-12. The c subunit is a small hydrophobic polypeptide, consisting almost entirely of two trans-membrane helices, with a small loop. These subunits are arranged in two concentric rings. The ε and γ subunits of F1 form a leg-and-foot that projects from the bottom side of F1 and stands firmly on the ring of c subunits.
Mechanism of Energy Transduction: Theory # 4.
Binding-Change Mechanism of ATP Synthesis:
The most widely accepted mechanism of ATP synthesis is the so-called binding change mechanism proposed originally by Paul Boyer.
The key feature in this mechanism is that the conformational changes in the protein are critical. According to this mechanism, the energy stored in the proton gradient is not directly used to drive the synthesis of ATP but rather is used to release a tightly bound form of ATP from the active site of the enzyme.
The three active sites of F1 take turns catalysing ATP synthesis. Each β-subunit can exist in one of the three distinct conformational states — loose (L), tight (T) and open (O). At any time, all three states are present in the CF1 complex, each being associated with any of the three P subunits (Fig. 9.23).
ADP and Pi initially bind to an unoccupied site in the open form. The conformational changes are driven by the passage of protons through the F0 portion of ATP synthase. The movement of protons through the F0 “pore” causes the cylinder of c subunits and the attached γ subunit to rotate about the long axis of γ, which is perpendicular to the membrane plane. The γ subunit passes through the centre of the α3 β3 spheroid.
The spheroid is held stationary relative to the membrane surface by the b2 and δ subunits. With each rotation of 120°, γ comes into contact with a different β subunit, and the contact forces the β subunit into the open conformation with the movement of protons.
The three β subunits interact in such a way that when one takes the open conformation, its adjacent one subunit must take the loose form, and the other adjacent subunit takes the tight form. Thus, one complete rotation of the γ subunit causes each β subunit to cycle through all three of its possible conformations, and for each rotation, three ATPs are synthesized and released from the surface of the enzyme.