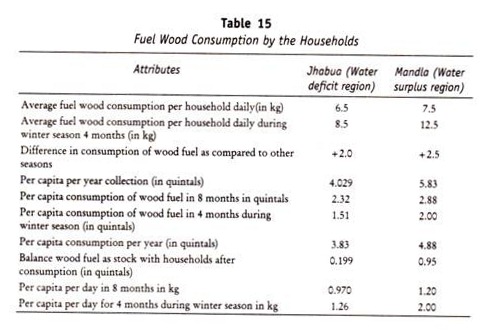
The below mentioned article provides a close view on the plant movements. After reading this article you will learn about: 1. Classification of Plant Movements 2. Geotropism 3. Phototropism 4. Explanation of Phototropism and Geotropism by the Hormone Concept 5. Hydrotropism 6. Chemotropism 7. Haptotropism or Thigmotropism 8. Nastic Movement and 9. Movements of Leaflets of Mimosa Pudica.
Movement is not usually associated with plants and least of all with trees or shrubs. If we think of movement in the narrow sense of an entire organism trans-locating, then we find very few instances in plant world. Perhaps, the best known cases are when a unicellular alga such as Chlamydomanas moves towards light or when spermatozoids move towards the egg cell by means of flagella. Many locomotion’s are, however, automatic and do not depend on any external stimulus such as shown by plasmodesmata of Myxomycetes or amoeboid movements which are really creeping movements due to some internal stimulus.
Flagella or cilia are locomotive organs, whip-like in shape which the organism moves to and fro by a process of contraction and expansion which recalls, but is really quite different from, the muscular contraction of animals. Locomotion’s of this type are shown by ciliated plants, gametes and zoospores while the plasmodia of Myxomycetes display creeping, amoeboid movement.
If on the other hand we think movement in the sense of a part of an organism moving due to unequal growth rates of the opposite side, then the examples in the plant world are numerous. Parts of the plants, their roots, their leaves, their stems all are capable of movements and do in fact move continuously.
Plants possess irritability and execute movements in response to a variety of external stimuli which act on protoplasm of the cell. Such movements can only be induced because the stimuli act on an excitable protoplasm. Some purely mechanical movements, which have nothing to do with protoplasm, are of common occurrence in plant kingdom as when sporangia or fruits dehisce in the dispersal of spores and seeds and in the coiling or uncoiling of elaters as in Equisetum. These are purely hygroscopic movements, largely determined by the presence or absence of water.
It is often customary to refer to autonomic movements. These are movements which are apparently independent of the surroundings of plants, i.e., of any external stimulus. A rough analogy in a man would be the continuous beating of heart which is quite independent of the environment as opposed to the response of the hand which withdraws when it is scorched. In the latter case, an external stimulus causes the movement. Perhaps the best known example of autonomic movement in plants is the movement described by the apex of the stem of many plants.
In plants such as peas or beans, it will be observed that the stem apex regularly describes a circular, spiral type of growth movement. This circular movement which is quite independent of any external conditions is always in the same direction for the same plant species. The more rapid rate of growth travels around the tip which, as it grows upwards must therefore rotate.
The immediate cause is that the growth on the different sides of the stem differs. First one side grows faster, then the other, and this goes on in a continuous regular alternation. This type of movement is referred to as mutation, preferably circumnutating. Similar type of movements is shown by the tendrils of many plants.
The second type of movement which we usually meet in plants is that which takes place in response to changes in the environmental conditions of the plant. The bending of aerial shoots grown near a window towards the light, the downward growth of roots, etc., all these exemplify the responses to changes in the surroundings.
These are referred to as tropisms because there is a directional relationship between the response and its causative effect—response is either towards the stimulus which is acting unilaterally from one direction only or away from it or at a definite angle to the direction of the stimulus.
There is another kind of movement (nastic movement) in which the stimulus is diffuse, i.e., it does not affect the organ from any definite direction and the movement, thus induced by the stimulus, is determined exclusively by the properties of the irritable cells. The response of the organ here is independent of the direction of the stimulus. Examples are afforded by many flowers which open when temperature rises and close when it falls. Pulvinar movements such as shown by the sensitive leaflets of Mimosa pudica are always nastic in nature and determined by the properties of the pulvini.
The environmental factors responsible for the growth difference are definitely known and are spoken of as the stimulus. The length of time to which the plant or the plant organ must be continuously exposed to a given intensity of stimulus for a visible response to follow is called the presentation time; the period from the commencement of stimulation to the commencement of the reaction or response is termed reaction time and the time it takes for the plant to recover its original position, after the stimulus has been removed, is the relaxation time. An organ may also perceive a stimulus but may be unable to react to it.
Movements can be classified in various ways. The classification which the biologist employs is largely a matter of convenience by which certain groups of plant behaviours can be related to each other. The differences in terminology are again based on convenience rather than on principle.
Classification of Plant Movements:
A. Purely Mechanical movements unrelated to irritability of the protoplasm-movement associated with dehiscence of sporangia (e.g., fern; Fig. 750), with explosive bursting of many fruits, dispersal of spores and seeds, movements of elaters (e.g., Marchantia and Equisetum) and so forth.
Tropic Movements:
In case of tropic movements, as we have seen before, the direction of movement shows a definite relation towards, away from or at a definite angle, to the direction of the stimulus.
The curvature developed by an organ, when its preferred orientation with respect to an environmental factor is disturbed, may be called a ‘tropic response’. The environmental factors only act as triggers which alter the pathway of metabolism in such a manner that the energy-delivering system of metabolism enables the response to take place.
The environmental or the external factor is called a stimulus—the stimulation process itself, i.e., the very earliest changes inside the organism which are brought about by the effect of the external factor is still little understood. The processes could be subdivided into subsection and perception (reception), i.e., purely physical processes and the immediately following physiological ones respectively.
A movement towards the stimulus is called a positive movement, away from the stimulus, a negative movement. The terms geotropism, phototropism, hydrotropism, chemotropism and haptotropism (or thigmotropism) describes movements induced by external stimuli such as gravity, light, water, chemical substances and contact or touch respectively.
The terms are described below:
Geotropism:
The stimulus of gravity must be unilateral since the force is exerted from one direction only. Primary roots are positively geotropic (i.e., bend towards the centre of the earth), primary stems are negatively geotropic; as a matter of fact all vertically upward- growing organs, whether stems, leaves, flower stalks, parts of flowers and even roots, such as the respiratory pneumatophores of many halophytic plants, are certainly negatively geotropic. When such organs are forced out of their upright position, they may assume it again if they are still capable of growth.
Secondary lateral roots and shoots show a weaker response or no response at all and take up a position at an angle to the gravitational force. A spreading fibrous root system as in monocotyledons is indicative of very feeble geotropic response. Secondary lateral roots are said to be plageotropic and are directed downwards and laterals of higher orders, both roots and shoots are practically insensitive to the stimulus of gravity (apogee-tropic). Rhizomes or runners below ground or on the surface, grow horizontally involving only a positional response that is they take up a position at right angles to the direction of gravity (diageotropic).
The adventitious, pneumatophores of halophytes are negatively geotropic and negatively hydrotropic but seem positively aero-tropic.
The response of an organ to the gravitational stimulus may change with the stage of development. The peduncle of a poppy flower bud is directed downwards, i.e., positively geotropic, but it gradually becomes negative as the flower opens.
The first sign of geotropic response occurs at a short distance away from the apex (Fig. 752), except in monocotyledons, in which case it occurs at the nodes where the meristems are found.
The geotropic responses are of universal occurrence. When an entire plant is placed horizontally its stem will quickly go upwards and its roots downwards. It was at one time thought that the downward curvature of horizontally placed roots resulted solely from their own weight. But it is now cell established that geotropic curvatures are possible only through growth. Roots when they execute positive geotropic movements penetrate mercury, (Fig. 753) a liquid in which dead roots float. That gravity is the operative stimulus in geotropic curvature can be easily shown if we expose all sides of the plant equally to gravity.
This is done by placing a plant horizontally on a wheel which slowly revolves in the vertical plane around a horizontal axis in an apparatus known as clinostat (1-4 rotations per hour). In this way, the plant is slowly turned so that each side in turn is exposed to gravity to the same extent (Fig. 754). When this is done, the plant or its parts no longer shows any upward or downward curvature. It just grows as it is placed— straight along (Fig. 755).
If, however, germinating seedlings are fastened in all possible positions at the periphery of a wheel and the wheel rotated very rapidly on a horizontal axis, it is found that the roots grow radically away from and all the shoots radically towards the centre of the wheel. As effects due to gravity were the same time considerable centrifugal force was produced) the centrifugal force determined the curvature of the seedlings, as gravity does normally.
The geotropic and phototropic movements are due to unequal growth of the two sides of the organ which is again caused by an unequal distribution of native auxin. The gravitational stimulus must be of certain minimum length of time and intensity in order to cause the response. The exact nature of perception of gravitational stimulus is, however, less clear, but attempts to explain the pheonomenon in terms of sensory cells (statocysts) and statoliths have achieved remarkable results during the last few years.
Many plants contain in certain cells of root tips, large starch grains (amyloplasts) which move under the effect of gravity within these sensory cells—statocysts. These movable starch grains were for a long time (since 1858) supposed to be receptors of gravitational stimulus or impulse. Recent observations during the last decade completely confirm the idea inherent in the classical works of Haberlandt and others (1900).
Starch grains or amyloplasts normally form an integral part of the graviperception mechanism in plant organs and the basic concept of statolith theory is now generally accepted. However, mitochondria and dictyosomes also respond to gravitational stimulus and the same is also true for other cellular micro-bodies.
Since dictyosomes and vesicles formed by them seem to be in some way connected with metabolism of cell wall the components and the formation of cell wall itself, and geotropic curvature involves differential auxin-induced growth which in turn implies an indirect interaction of auxin on cell wall components, dictyosomes may play an important role in geotropic curvature.
Phototropism:
As a rule, stems are positively phototropic whereas leaves are plageophototropic or transversely phototropic (Fig. 756). The advantages gained by these responses are that assimilating parts are brought to a position where light is plentifully received. Study of many leaf-mosaics shows surprisingly little overlapping of leaves. The majority of roots are insensitive to light.
Only in a few species, one of which is white mustard (Sinapis alba) do the roots show the reaction one might usually expect, namely, negative phototropism. The peduncles of some flowers are positively phototropic after fertilisation is over. The effect of unilateral light stimulus may be neutarlised by rotating the plant on a vertical clinostat. No phototropic curvature then occurs.
The phototropic phenomenon is a very intricate and fascinating mechanism. The reaction to light is certainly quantitative. A certain minimum of light is definitely required to cause a response. This minimum of light must be given for a certain period of time. There seems to be a strict relationship between the period of illumination and the light intensity.
Illumination causing a definite known response in plant as shown by the bending of a stem or a coleoptile by a measurable number of degrees, will in every case be the same if the illumination is for 100 seconds with 10-foot-candles or for 10 seconds with 100-foot-candles as with 1,000 seconds with one-foot-candle. Thus a given amount of energy produces A given amount of photo- tropic curvature and the product of the light intensity and the period of illumination needed to bring about a given phototropic response, is constant.
Time period, however, cannot be reduced indefinitely; therefore is a minimum presentation time during which the light must be given to the plant for it to be perceived. However, both the minimum time and the minimum light needed to cause response are very small. The time may be as little as fraction of a second and the minimum amount of light required not much more than the light from an ordinary torch (one-foot-candle).
These clear-cut and simple light responses do not, however, hold good when high light intensities are reached and then there is no longer a straightforward relationship between response and intensity of illumination as indicated above. Thus if a coleoptile tip is illuminated for 1 min. with 1,000-foot-candles, we certainly do not get 10 times the response given by 100-foot-candles for 1 min. nor again does the latter give 10 times as much as 10-foot-candles for 1 min.
In fact the response in the form of the extent of curvature becomes progressively smaller as the light intensity rises till saturation point is reached. There is then no further increase in the response by increasing the intensity of light stimulus. Indeed if the light intensity is very high, the plant or the coleoptile tip till will move away from the light and not towards it.
As in the case of geotropism, it is easily shown that the region of perception and the region of response to the light stimulus are not the same. The sensitive zone is the first 1-1.5 mm from the tip.
In the phototropic response of dicotyledonous leaves the question of perception of stimulus is interesting. In the peltate leaf of Nasturtium, lateral illumination of the whole leaf produces a strong positive curvature of the petiole. Removal of the lamina drastically reduces the extent of the response.
On the other hand, screening of the lamina with black paper hardly impairs the positive curvature of the petiole while the lateral illumination of the lamina with only the petiole shaded, produces no curvature at all. Thus it is apparent the light stimulus is perceived by the petiole only. The leaf blade appears to act as a natural source of auxin for the petioli, enabling the petiole to carry out the growth curvature.
The wave-lengths that induce phototropic response towards a unilateral light are certainly not identical with those involved either in photoperiodism or photosynthesis. The maximum, as we have seen before, is found in the blue, in the neighbourhood of 450 nm with a minor peak at 480 nm. Phototropic curvatures, however, have been observed in response to much shorter wave-lengths, far into violet. The photoreceptive pigment is probably a flavone derivative, e.g., riboflavin (vitamin B2) or it may conceivably be β-carotene, or some other carotenoid.
Explanation of Phototropism and Geotropism by the Hormone Concept:
If a coleoptile tip is exposed to unilateral light, it results in an unequal distribution of hormones on the two sides of the tip and it can easily be shown, by collecting the hormone diffusing out into agar blocks from the two sides, that the dark side always has more hormone than the side which is illuminated.
It seemed pretty obvious that light causes the destruction of hormone on the illuminated side of the plant. If this is so, then in the tip of the oat coleoptile or for that matter in any sensitive region illuminated hormones appear in different amounts on opposite sides of the tissue. Other possibilities of this unequal distribution of hormone in different amounts on the two sides are: (i) that there may be a light-induced transverse migration of hormone from the lighter side to the darker side or (ii) the sensitivity of the tissue on the lighter side to hormone may be decreased. In any way, in an illuminated coleoptile, the darker side certainly contains more hormone than the lighter side. The hormone then diffuses downwards and reaches the zone of response in unequal amounts, causing different rates of cell elongation which in turn causes curvature to take place.
For light to be effective in any system like this it must first be absorbed to bring about a photochemical reaction, leading to the destruction or redistribution of auxin. Since hormones are colourless compounds, it is clear that hormones cannot be the absorbers of visible light, which is effective in producing phototropic curvature. It has definitely been established, we know, that not all light is equally effective in causing phototropic curvature.
Blue light is very effective in causing the movement, while red light is almost ineffective, the response to other colours of light being intermediate of the two. Of the pigments which are present in the oat coleoptile only two are so far known to be active in the absorption of blue light.
They are yellow pigments, vitamin B2 (riboflavin) and carotene. The candidatures of both are strong. The action spectrum (the curve relating action, i.e. phototropic curvature to wavelength of light) of phototropism exhibits peaks at about 360 nm and 445 nm with shoulders or minor peaks at 425 and 472 nm. Riboflavin does have an absorption maximum at about 360 nm, but β- carotene has no absorption in this region; on the other hand the absorption peaks in the region 400 nm and 500 nm are shown β -carotene whereas riboflavin has only one broad peak at about 460 nm.
The absorption spectrum of none of the pigments thus corresponds reasonably with the action spectrum of phototropism. However, the absorption spectra of isolated pigments may not be identical with the native bound form and thus, either of the pigments or both acting co-operatively may be the photoreceptor pigments.
However, it should be pointed out that a strain of the fungus Pilobolus produces sporangiophores (Fig. 757) which contain no carotene at all but these sporangiophores which are completely carotene-free appear to have retained their full phototropic responsiveness; they seem indeed even more sensitive than the normal ones with carotene. Other flavins may also be involved.
It is now generally, believed, and there is good evidence for this, that the photo-tropic curvature is due to lateral transport of auxin from the lighted to the shaded side, and not due to any marked destruction of auxin. In vitro studies however, show that riboflavin may catalyse photolysis of IAA.
Photosensitiser substances are of frequent occurrence in the living cells including plant cells. Riboflavin absorbs blue light and the light energy thus absorbed by the photoreceptor pigment for the phototropic reaction, is thought to be utilised for the photochemical destruction of IAA in the lighter side thereby bringing about the unequal distribution of hormone in the two sides of the coleoptile.
What about the other yellow pigment, carotene, whose absorption spectrum considerably coincides with the action spectrum of phototropism? Can it bring about a similar photolysis of IAA as riboflavin? Available evidence indicates that it is not so. Using aqueous mixtures of IAA and colloidal solutions of carotene or using true alcoholic solutions of both, no photolytic destruction of IAA could be discovered in visible light.
Another possible effect in a different direction which could be produced by the presence of carotene in the system has been examined and the most impressive discovery was that the addition of carotene to the reaction system, IAA+ riboflavin, almost completely inhibits the photo-catalytic action of riboflavin on IAA.
This protective effect seems to be chiefly due to the similarity of the absorption spectra of both the yellow pigments in the critical region where they largely overlap. Carotene may also act as an internal light-filter screening off the most effective wave-length necessary for photo-inactivation of IAA by riboflavin thereby causing a suitable drop in the light intensity across the coleoptile.
It seems certain that there is a strong competition for light between riboflavin and carotene and if sufficient light is diverted for absorption by carotene, riboflavin can no longer mediate the destruction of hormone. Without carotene, light would reach both sides of the coleoptile in equal amounts as the coleoptile sheath is only about 1.5-2 mm thick. Therefore, the presence of protective carotene filter in the coleoptile could reduce the transverse light gradient, thereby progressively increasing the hormone content from the lighted to the dark side.
Many of the observations which have been described for phototropism are also true for geotropism. Thus, the geotropic movement is due to unequal growth which is caused by an unequal distribution of hormones. If an Avena coleoptile tip is placed horizontally, the lower part is found to contain as much as 66% of the total hormone content of the tip.
But why should gravity cause such an unequal distribution of auxin? It is worth pointing out here again that reaction of roots to hormones differs from that of stems and the important thing to remember is that while in the stem, the side containing most hormone grows fastest, the opposite is the case in that root.
Thus it must be postulated that the reaction of tissues to particular concentration of hormone is different in the two types of tissues, the shoot and the root. Whereas a particular concentration of hormone induces acceleration of growth in the short end, the same concentration actually produces an inhibitory effect in the growth and enlargement of the root cells. This explains why when a plant is placed horizontally, the stems turn up and the roots down even in absence of light.
The hormone in this case concentrates on the lower side of the plant (actually growth inhibitors have been identified by chromatographic separation from the upper part of the horizontally placed mesocotyl— the nodal zone between the grain and the coleoptile of maize) resulting in more rapid growth of the cells of the lower side of the stem tissue causing them to bend upwards and faster growth on the upper side of the root tissue, causing roots to bend downwards.
Hence the overall geotropic response is obtained. It has been suggested that the reason for the unequal distribution of auxin in the horizontally placed plant is partly due to the creation of a potential difference across the plant, the lower side becoming positive. This may cause an accumulation of hormone on the lower side of the plant because of the polar flow towards the positive pole, i.e., lower side.
Hydrotropism:
In certain localities, soils do not contain water uniformly; some parts may be wetter than others. Primary roots tend to grow towards the wetter regions. The positive hydrotropism of roots may be demonstrated by growing plants in moist sawdust in a shallow sieve, inclined to the vertical (Fig. 758).
Hydrotropism of roots can also be demonstrated as in Fig. 759. The roots grow vertically downwards due to the stimulus of gravity until they penetrate the sieve. They then bend back by growth curvature from the dry air outside towards the moist sawdust. Apparently hydrotropic stimulus is more powerful than gravitational impulse.
Chemotropism:
Chemical substances in the environment have a directive influence on the growth of certain plant structures. The classical example is afforded by the growth of the pollen tube through stigma and style towards the embryosac cell. It is presumed that chemical substances present in the carpels are the directive force for the growth of the pollen tube.
Calcium and boron are the major chemical substances responsible for this movement. When pollen grains germinate in nutrient jelly in which pieces of carpel have been sown, generating pollen tubes grow away from the air and move towards the pieces of carpel; the pollen tubes show negative aero-tropism and positive chemotropism.
The first orientation of germinated pollen tubes into the stigma takes place by hydrotropism, followed by a mechanical conduction all along the slimy way. For the final part of the movement of pollen tube through the style, there is no doubt that chemotropic orientation is responsible (Schneider, 1956).
The sucking roots of parasites and hyphae of parasitic fungi penetrate into the tissues of the host plant in response to the stimulus of perhaps other chemical substances contained in them. Movements of individual tentacles of Drosera in response to various chemical substances, both inorganic and organic (in the form of insects), placed in the leaf blade are also examples of chemotropism.
A portion of the chemical substance is absorbed by the leaf, protoplasm is stimulated and a semi-motor impulse goes from the leaf to the bases of surrounding tentacles which bend down on the substance or the insect on the leaf blade. Thus glandular heads of the tentacles are brought in contact with the insect and the organic matter can be digested and assimilated by the plant with the help of excreted enzymes.
Haptotropism or Thigmotropism:
The petioles of many species of plants, the leaf tip of Gloriosa, specialised tendrils of garden pea, the stipules of Smilax, the branch of vine, etc., are sensitive to contact with the uneven surface of solid bodies and execute haptotropic curvatures. One of the most remarkable facts is that these structures while sensitive to contact with the surface of a glass rod, is insensitive to that of a glass rod coated with gelatine gel (i.e., a colloidal emulsion). Raindrops, again a liquid, seem to inhibit haptotropic curvature.
It appears that haptotropism results from compression of cells on the stimulated side (i.e., the side in contact with the foreign body) of the sensitive structure while the growth of the opposite side continues. A short curvature is thereby produced and the sensitive structure coils round the support with which it makes contact (Fig. 760).
Though the movement of the individual marginal tentacles of Drosera towards the body of the insects has rightly been described previously as chemotropic movement since the inducing stimulus has a directional influence, the first movement of a marginal tentacle towards the small central tentacles, on the other hand, can only be regarded as chemonastic or even haptonastic. This movement is exclusively determined by the properties of the tentacle for the stimulus exerts no directive influence.
Nastic Movement:
When an external stimulus induces a certain process or influences its intensity without attaching any significance to the direction of the stimulus, we have nastic movements. In nastic movements, the direction of curvature is, as we know, morphologically pre-determined. As in the case of tropic movement, nastic movements can be brought about either by unequal growth on the opposite halves of the organ or simply by turgour changes.
Diverse forms of biological advantages may result to the organism from nastic movements. The chemonastic movements shown by insectivorous plants lead to the assimilation of nitrogenous food.
The commonest of nastic movements are nyctinastic movements, the day-and-night movements of leaves and flowers. By nyctinastic growth curvatures, certain flowers can perform opening and closing movements. Perianths and many compound leaves may open during the day and close at night. An interesting nastic movement is observed in some species of cactus; the flowers only open in the dark. This is also true for some tobacco flowers.
Some of the nastic movements depend on turgour changes rather than on differential rates in growth: Families, typically showing nyctinastic leaf movements are Leguminoseae, Oxalidaceae, Euphorbiaceae and also pteridophytic species of ferns and Marsilia. The night position of shoot is always characterised by a vertical position of the leaf blade, the petiole curving either upwards or downwards. In the day position the leaf blades stand horizontally or at right angles to the incident light.
Nyctinastic movements may be controlled by temperature and light. These individual factors acting singly may induce photonastic and thermonastic movements respectively. The petals of tulip and crocus open at a constant temperature when illuminated and close when darkened. If on the other hand, the intensity of light is kept constant, they open at high temperature and close at low temperature. They are, therefore, both photonastic and thermonastic. In each case, the opening is the result of growth curvatures resulting from more rapid growth of the upper surface (epinasty).
When the lower surface grows more rapidly (hyponasty), growth curvature resulting in closure follows. The rolled circinate vernation of young fronds of fern is due to epinastic growth curvature; its subsequent straightening up is due to hyponasty. Nastic movements are also caused by IAA (auxin epinasty) as well as du6 to the presence of certain substances in minute traces; ethylene present in concentration of 1: 500,000 parts of air may cause epinastic movements of tomato leaf petioles.
Nyctinastic movements in some cases appear to have ecological significance in protecting the young leaves or developing flower buds from injury due to light, high temperature and especially by rain. It must be admitted, however, that in most cases, they are in part useless movements.
Movements of Leaflets of Mimosa Pudica:
Perhaps the most striking of all plant movements is that of the semimonastic movements of the sensitive plant, Mimosa pudica. This is also sometimes referred to as haptonastism or thigmonastism. The plant is sensitive to touch, impact, electrical impulses and heat. While the tropic movements are generally, slow taking many minutes, the response of the sensitive plant to any form of shock is almost instantaneous.
Mimosa has compound leaves made up of leaflets which in turn are made up of pinnules. When the plant is stimulated by touch, the pinnules begin almost immediately, within seconds, to fold up. If the stimulation is vigorous, this is followed lip by drooping of the whole leaflets at the junction of the petiole.
When a really vigorous stimulation is provided, as for example applying a match to the tip of one of the pinules, the petiole itself droops at its junction with the stem and this is followed by the closing and drooping of all the neighbouring leaves till the leaves of an entire branch have completely folded up.
The whole process is extremely fast and within a minute all the leaves of the branch will have closed. The really important thing is the sequence of closing; in the stimulated leaf, first the pinnules, then the leaflets and finally the petiole close up. In the neighbouring leaves, however, the process is completely reversed. Here the petiole drops first followed by the leaflets and only at the last stage do the pinnules fold up in pairs.
The movement of the leaflets of Mimosa is affected by external conditions such as temperature. The leaflets pass into a cold rigour at low temperature and into a heat rigour at about 40°C. The leaflets lose their power of movement in continued scarcity of water and also in continued darkness. The power, of movement is lost also under anaerobic conditions.
The tropic, nastic and various other movements previously described are, as we have seen, caused by differential growth. The movement of Mimosa is, however, very different in mechanism. The pinnules are connected to the leaf-rachis and the rachis is connected to the petiole by swollen leaf bases which are called pulvini (Fig. 752). These pulvini contain cells chiefly situated in the lowest half which normally are completely filled with water, i.e., they are fully turgid.
These turgid cells of the pulvini, however, may readily lose their water to the neighbouring cells and intercellular spaces, as actually happens, due to shock or touch stimulus. When therefore a sudden loss of water occurs from the cells, the lower half of the pulvinus loses its previous rigidity and can no longer support the weight of the leaflet or leaf attached to it which as a result droops downloads.
In view of the extreme rapidity of the response of Mimosa to shock or touch stimulus which almost suggests the nervous response in animals, it seems that a chemical substance is being translocated through the plant very rapidly. There is more than one way of transmitting this shock stimulus. One is very fast and passes in one or two seconds from the pinnule stimulated, to the main petiole pulvinus.
This pathway exists only through living cells (primarily through phloem) of the plant and it has been proved that this stimulus cannot be transmitted through dead tissues. The second, however, is somewhat slower, taking up to a minute or two and it can certainly be transmitted through the dead tissue. The second pathway is apparently the mode of transmission from one compound leaf to the other.
Several workers (1938-1957) have made attempts at the purification and determination of the chemical structure of this stimulating substance in Mimosa, but so for none has succeeded. An amorphous concentrate, extracted from Mimosa, gives the pinna response, at a dilution, 1.5 X10-8. This substance behaves as an oxyacid, containing nitrogen (4-5%), with an estimated molecular weight, between 300-450.
Most of the literature concerning the mechanism of rapid movements in plants, particularly Mimosa, concludes that the movements are caused by the diminishing or sudden loss of turgour in the motor cells of the pulvini or decrease in their volume or both. Expulsion of water from the main pulvinus during movement and reabsorption of water during recover have been seen by the use of micropotometer.
Much of the recent evidences do clearly indicate that the motor cells in Mimosa, either in pinna or in pinnules, have contractile vacuoles, whose activity causes liquid— probably cell sap—to be expelled from the motor cells, just like the contractile vacuoles seen in Protozoa; this activity might conceivably result in decrease in turgour or the volume of the motor cells. This has nothing to do, however, with the increase in permeability of the plasma membrane.
In 1960, Aimi proved that the sensitive motor cells exist even in the upper-half as well as the lower-half of the main pulvinus. His conclusions were that the magnitude and direction of the petiolar movement in Mimosa is expressed by two forces antagonising each other, each of which consists of bending force, due to the contraction of one-half and tissue tension in the other; normally this force, in a downward direction, would be much greater than the upward force. Some differences between the two halves of the pulvinus can actually be observed in the morphology and the activity of the cells—the walls of the cells of the upper-half are actually significantly thicker than that of the lower.
The enzyme ATPase has been identified in the young pulvini but the significance of its presence is still unknown. It may be that the activity of contractile vacuoles or proteins depends on a mechano-chemical reaction caused by an ATP-ATPase system.
How the liquid, which has been expelled into intercellular spaces during response, re-enters the cell interior during recovery is still unknown. An active accumulation of salts or ions, particularly potassium, has been detected in the intercellular spaces of Mimosa petiole and the presumption is that, ATP, working the K-pump takes part in the recovery process of the motor cells, i.e., the re-entrance of the liquid from exterior to the interior of the cells.
It must be admitted that the sensitiveness and the accompanying somewhat complicated motor movement of the leaflets of Mimosa do not appear to serve any obvious useful purpose to the plant.