In this article we will discuss about Cytokinins. After reading this article you will learn about: 1. Structure of Cytokinins 2. Biosynthesis of Cytokinins 3. Plant Responses 4. Mode of Action.
Structure of Cytokinins:
Skoog, Strong and Miller proposed the definition of cytokinin. It is a compound which besides other activities induces cytokinesis, i.e., cell division in the various plant organs grown in a medium containing an optimal concentration of auxin.
The name ‘kinin’ was originally used for this plant cell division promoter, but this name led to some confusion with the same term used by the animal physiologists. So, the name kinin has been replaced by ‘cytokinin’. All the naturally-occurring cytokinins are substituted purines. The usual way of naming a cytokinin is to express it as a substituted 6-amino purine or as N6-substituted adenine.
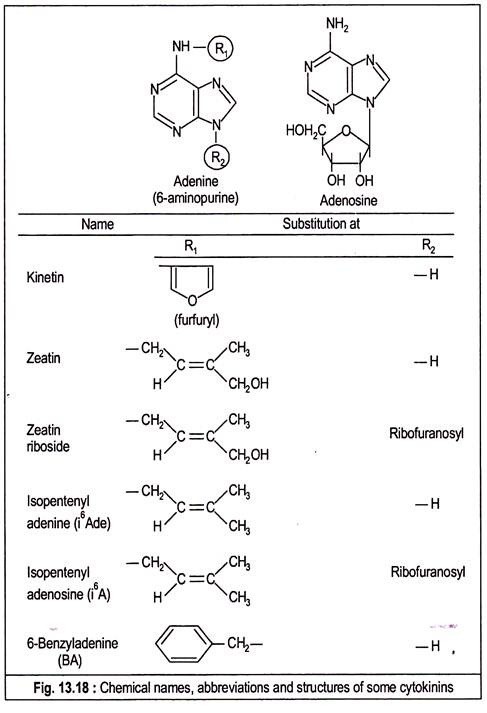 In 1956, Miller isolated the first cell-division inducing factor from autoclaved herring sperm DNA which they named kinetin and identified as 6-(furfurylamino) purine. It was later revealed that kinetin is not a natural component of DNA but is its breakdown product. Synthetic kinetin is also available as a powerful promoter of cell division.
In 1956, Miller isolated the first cell-division inducing factor from autoclaved herring sperm DNA which they named kinetin and identified as 6-(furfurylamino) purine. It was later revealed that kinetin is not a natural component of DNA but is its breakdown product. Synthetic kinetin is also available as a powerful promoter of cell division.
In 1963, Letham isolated 6-(4-hydroxy-3-methylbut-trans-2-enylamino) purine from immature kernels of Zea mays and named it ‘zeatin’.
Other cytokinins identified from the same source are zeatin riboside and its 5-phosphate derivatives with one or more phosphate group, i.e., zeatin ribotide. Both zeatin and zeatin riboside have been identified in the culture filtrates of mycorrhizal fungus Rhizopogon roseolus. Dihydrozeatin with a saturated side chain has been identified from higher plant.
Other active cytokinins widespread in plants are N6, N6-dimethylallyIaminopurine or N6 (Δ2– isopentenyl) adenine (i6ADE) and its ribosyl derivative N6 (Δ2-isopentenyl) adenosine (i6A).
Culture filtrates of bacteria like Corynebacterium fasciens causing fasciation disease and Agrobacterium tumefasciens producing crown gall have been shown to contain both i6ADE and i6A which have also been synthesized. Another cytokinin 2-methylthio-N6 (Δ2-isopentenyl) adenosine (ms2i6A) has been identified from yeast.
After kinetin, a large number of analogous compounds including those just mentioned were synthesized by replacing the N6 side chain with a variety of groups. Very active synthetic cytokinin is 6-(benzyl amino) purine or benzyl adenine which together with kinetin are largely used in laboratory experiments. Substitutions at other positions of the purine nucleus generally result in compounds with lower bioactivity.
Cytokinins in tRNA:
Cytokinins are structural components of transfer RNA (tRNA) present in organisms ranging from bacteria to man while these are not present in ribosomal RNA (rRNA).
Such substituted adenines are located as an odd base immediately adjacent to the anticodon of different tRNA species like serine tRNA (tRNAser), phenylalanine tRNA (tRNAPhe), tyrosine tRNA (tRNAtyr), leucine tRNA (tRNAleu) and a few others which are specific for these amino acids.
It has been further shown that a cytokinin is associated with the tRNA corresponding to each codon with the initial letter U. On the other hand, tRNAs that translate codons with initial letters other than U do not show activity. Since certain tRNAs contain cytokinins, one can expect that turnover of tRNA serves to provide a source of free cytokinins.
It is, however, doubtful whether tRNAs with cytokinin-active bases are able to meet the demand of hormonal cytokinins. Some arguments have been made in support of the hypothesis that tRNA cytokinins should not be regarded as functional cytokinins. First, tissue cultures, which require external cytokinins for growth and proliferation are expected to contain cytokinins in endogenous tRNA.
Secondly, certain cytokinins that are found as free compounds in plants are not found in the corresponding tRNA. Thirdly, zeatin like cytokinins in plant tRNA have cis-configuration of the side chain, whereas the majority of active cytokinins have Trans-configuration. These evidences lend support to the idea that tRNA is not a major source of cytokinin.
Biosynthesis of Cytokinins:
Two mechanisms have been proposed for cytokinin biosynthesis, one leading to tRNA cytokinins and the other to free cytokinins. Since certain tRNAs contain cytokinin, synthesis and turnover of tRNA is the possible route to free cytokinin formation. Evidences suggest that biosynthesis via tRNA may not be a major source of cytokinins.
So, it seems unlikely that any significant amount of free hormonal cytokinin in plants is derived from the degradation of tRNA. There is also evidence that free cytokinins do not take part in the synthesis of tRNA cytokinins. On the contrary, tRNAs are first made by conventional method and then polymerized into the final tRNA molecule.
After this, the isopentenyl groups are transferred from isopentenyl pyrophosphate (IpPP) by an enzyme known as prenyl transferase which belongs to a group of enzymes required for the synthesis of other isoprenoid compounds.
Such prenyl transferase which synthesizes tRNA cytokinins does not utilize free AMP as a substrate, rather it has the property to recognize a specific base sequence in the tRNA molecule and transfer the isopentenyl group to the adenosine adjacent to the 3′ end of the anticodon.
Free cytokinins are synthesized in a different manner. For the direct synthesis of free cytokinins, an enzyme called isopentenyl pyrophosphate: AMP isopentenyl transferase, also known as cytokinin synthase catalyzes the conversion of 5-AMP and isopentenyl pyrophosphate into i6ADE.
It has also been noticed that cytokinin synthase involved in the synthesis of free cytokinins is also a type of prenyl transferase but is not the same transferase that is involved in the synthesis of tRNA cytokinins. Although the i6ADE or its ribotide derivative are not the major cytokinins of higher plants, it can be readily converted to zeatin and other major cytokinins.
It has been observed that crown gall tumors of plants produced upon infection by the bacterium Agrobacterium tumefaciens, can grow in culture without the inclusion of auxin or cytokinin in the culture medium. This proves that crown gall tumor tissues contain substantial amounts of both auxin and cytokinin.
Agrobacterium contains a large, circular extra-chromosomal DNA known as the Ti plasmid. At the time of infection, a small portion of the Ti plasmid, known as the T-DNA is incorporated into the nuclear DNA of the host plant cell chromosomes. Genes necessary for the biosynthesis of auxin and cytokinin are carried by the T-DNA of bacteria.
It is only after crown gall transformation, a special class of nitrogen-containing compounds called opines are synthesized in plants. It is interesting to note that the opines are utilized only for bacterial nutrition and are of no use to the plants.
The T-DNA contains a number of genes for hormone biosynthesis. We know that an isopentenyl transferase enzyme transfers the isopentenyl group from IpPP to AMP to form the cytokinin, isopentenyl adenine ribotide. This transferase enzyme is encoded by ipt (isopentenyl transferase) gene that is one of the T-DNA genes.
Furthermore, the T-DNA contains two genes which encode the enzymes occurring in the pathway of tryptophan conversion to IAA, the native auxin.
Transport of Cytokinins:
When cytokinins are applied on leaves, only localized effects are produced. Likewise, cytokinins produced in fruits and seeds show no apparent movement. Such observations imply that the hormone is immobile in plants. On the other hand, the presence of cytokinins in the xylem sap proves that the hormone synthesized in roots moves upward in a polar fashion to the shoot and thereby regulates the growth of the plant.
It is possible that during active growth periods, cytokinins are synthesized in both root and shoot meristems. It is also evident that long distance transport can be envisaged in case of root only, whereas cytokinins produced in shoot meristem is distributed to the tissue lying close to the site of production.
Cytokinins are transported from the root to the shoot in the xylem as zeatin riboside. After reaching the leaves, some amount of these zeatin ribosides is either converted to the free base, which possess hormonal activity or to cytokinin glucosides. These glucosides are often devoid of hormonal activity possibly because these are compartmentalized within cells and are not available for physiological functions.
Plant Responses to Cytokinins:
Cytokinins play a prominent role in all the phases of plant development from cell division and enlargement to the formation of flowers and fruits. They increase resistance to aging and to adverse environment.
(i) Cell Division:
For continued in vitro growth and cell division of tissue accompanied by DNA synthesis, cytokinin is necessary along with auxin. While auxin and gibberellin are also able to stimulate DNA synthesis and mitosis, cytokinin alone can stimulate cytokinesis. Quite opposite to the pro-motive effect of auxin and gibberellin, cytokinin inhibits elongation of stem sections. Root growth is generally inhibited by cytokinins.
(ii) Cell Enlargement:
Cytokinins may stimulate radial growth of stem tissue by swelling rather than by longitudinal extension.
Vine well-known leaf enlargement caused by cytokinin is due to an effect on cell enlargement rather than cell division. In fact, cytokinin appears to promote overall enlargement of cells and not simply elongation. Cytokinin effect on cell enlargement may be due to an influence on micro fibril orientation from longitudinal to radial direction.
(iii) Tissue Differentiation:
Organs in tissue culture show a spectacular response to cytokinin. With a low cytokinin supply, the tissue remains as an amorphous undifferentiated callus.
Bud formation and shoot initiation depend on higher concentrations of cytokinin by changing cytokinin auxin ratios. An interesting observation on morphogenesis in tobacco callus cultures is that a high cytokinin auxin ratio results in the production of shoots but no roots, but a low ratio leads to an opposite effect producing roots only.
In addition to their role in leaf expansion, cytokinins also play a regulatory role in chloroplast formation. When cytokinin is absent, plastids are formed but remain undifferentiated. Presence of both light and cytokinin is necessary for grana development and conversion of pro-plastids into chloroplasts.
(iv) Retardation of Senescence:
The retardation of senescence by cytokinin is a well-known phenomenon. Richmond and Lang first discovered that when leaf discs are kept in water, senescence appears within a few days as evident by the loss of chlorophyll and protein. But when cytokinin is added to the leaf discs, senescence is delayed through the maintenance of chlorophyll and protein.
This senescence-retarding property of cytokinin as mediated through the retention of chlorophyll is known as Richmond-Lang effect.
(v) Mobilization of Nutrients:
Mothes observed that when a particular area of leaf is treated with cytokinin, that treated area remains green showing delay of senescence, while the untreated area loses its green colour and becomes yellowish showing symptoms of senescence. Here the nutrients are drawn or mobilized from other parts of leaf so that the treated area remains green at the expense of the untreated area.
(vi) Release of Dormancy of Seeds and Buds:
Applications of cytokinins can stimulate germination and break dormancy. One of the remarkable characteristics of cytokinins is their ability to modify the effects of other hormones without any marked effects by themselves.
When dormancy is imposed either by high temperature (thermo dormancy) or by an accumulation of inhibitor like ABA (inhibitor dormancy), then GA alone is not capable to overcome dormancy. Addition of cytokinin opposes the action of inhibitor and permits germination.
Thus cytokinin has been documented as a permissive agent in germination by antagonizing the inhibitor action — a case of cytokinin-inhibitor antagonism. In bud growth also, inhibitor (preventive) and cytokinin (permissive) show opposite effects. Thus inhibitor-induced bud dormancy can be overcome by cytokinin.
(vii) Apical Dominance:
Cytokinin applied on lateral buds is able to release them from apical dominance whether it is due to the presence of terminal bud or due to applied auxin. This has been interpreted as an increase in IAA transport and mobilization of metabolites from the apical region to the point of application of cytokinin which is supported by the striking influence of cytokinin on phloem transport.
(viii) Resistance to Adverse Factors:
Cytokinins increase the resistance of plants to adverse factors such as high and low temperatures and certain disease. The nature of the action of cytokinins in bringing about these effects is still unknown.
Naturally-occurring cytokinins have been implicated in host-parasite relationship. Infection by bacterium Corynebacterium fascines which produces cytokinin like i6ADE leads to the fasciation disease symptoms in many plants. Treatment with cytokinin may induce a similar pathogenic condition.
(ix) Stomatal Movement:
Cytokinin has a distinct action on the mechanism of stomatal movement. Although the stomatal aperture in the isolated epidermal systems is not much influenced by cytokinins, treatment of whole leaf with cytokinin has been reported to increase the stomatal aperture and thereby transpiration.
(x) Other Developmental Effects:
The development of inflorescence is influenced by cytokinin treatment by increasing both the number and size. Cytokinin has been shown to cause a male-flowering plant to produce hermaphrodite flowers. Enhancement of fruit set and fruit size in grape varieties and induction of parthenocarpy in fig have also been reported.
Mode of Action in Cytokinins:
(i) Control of Transcription and Translation:
At present, the biochemical basis of cytokinin action is still not completely known. Still there are many evidences which make it clear that cytokinins greatly influence nucleic acid metabolism. Guttman first reported that kinetin treatment of onion roots caused a rapid increase in nuclear RNA and this observation was later confirmed by Jensen et al.
A quick increase in the DNA of tobacco pith cells has been observed. However, it is not clear whether cytokinins exert any specific transcriptional control.
Both the chemical constitution of the cytokinins and their effect on nucleic acid synthesis strongly suggest that they exert their biological activity directly in nucleic acid metabolism. Presence of cytokinin activity in specific tRNA species would suggest the influence of cytokinins on specific rather than bulk protein synthesis.
Location of the cytokinins next to the anticodon in certain tRNA species with known base sequences suggests that they may function specifically in the translation step of gene- controlled protein biosynthesis.
Since various developmental pathways are influenced by cytokinins, it is pertinent to expect cytokinins to control the synthesis of many proteins either by regulating the transcription of the genes encoding these proteins or by an effect at the post-transcriptional level.
One well-known protein, the synthesis of which is induced by cytokinin is nitrate reductase enzyme. Nitrate reductase is a cytosolic enzyme, which reduces nitrate (NO3) to nitrite (NO2).
Further reduction of nitrite to ammonia (NH+4) is catalysed by the chloroplast enzyme nitrite reductase. It is interesting to note that nitrate metabolism is induced by light, which initiates the expression of both the nitrate reductase gene encoded by nucleus as well as the chloroplastic nitrite reductase gene.
Cytokinins can stimulate the synthesis of nitrate reductase in dark-grown leaves, which suggests that the light requirement for the induction of this enzyme can be partly substituted by cytokinins.
It has been further observed that along with the induction of nitrate reductase by cytokinin, there is a corresponding increase in nitrate reductase mRNA. Since cytokinin-stimulated nitrate reductase can be blocked by inhibitors of both gene transcription and protein synthesis, it may be concluded that cytokinin possibly exerts its effect both at the levels of transcription and translation.
(ii) Enzymes and Metabolism:
In cultured tissue, respiratory activity can be increased by the addition of cytokinins. It has been shown that cytokinin stimulation of respiration involves a suppression of glycolytic enzymes and a shift to the hexose monophosphate shunt.
In intact systems, high doses of cytokinins cause inhibition of respiration and delay of senescence can be correlated with decrease in respiration. Cytokinins have been found to influence the activity of a number of specific enzymes. Cytokinin induces the formation of tyramine methyltransferase which catalyses thiamine synthesis.
Although less effective, cytokinins may be substituted for GA in the induction of α-amylase in wheat seeds. In barley endosperm, cytokinins cannot replace GAs, but if the system is inhibited by ABA, the activity can be restored not by the addition of GA, but by cytokinin.
(iii) The Richmond-Lang Effect:
The Richmond-Lang effect suggested that cytokinins play an active role in senescence retardation in detached leaves. The mechanism is based on the postponement of the disappearance of chlorophyll and the degradation of proteins through the activity of the corresponding hydrolases which normally accompany the senescence process.
Mothes suggested that the primary function of cytokinin in this respect is to increase the amino acid accumulation and to increase or retain the protein content.
(iv) Identification of Cytokinin Receptors:
The fact that hormones can easily be taken up by plant cells suggests that these cells may be equipped with intracellular receptors. Specific high-affinity binding proteins have been described for nearly all major plant hormones. It is likely that the cytoplasmic cytokinin-binding sites represent such receptors.
In wheat germ, cytokinin-binding proteins (CBF-1 and CBF-2) have been characterized which appear to be either loosely bound to the ribosomes or present in free form in the cytosol.
A similar high-affinity cytokinin-binding protein (CBP) has been described by Polya ad Davis. The association of CBF and CBP with ribosomes suggests that these binding proteins might have a receptor function in the translation process.
It has now been established that the specific receptors with which cytokinins interact remain either on the cell surface or within the cytoplasm. Recently, a 67 kilo Dalton protein, designated zeatin-binding protein (ZBP), has been isolated from the cytosol of young barley plants. ZBP has a high affinity for zeatin, and zeatin binding is highly specific.
When ZBP-zeatin was added to isolated barley nuclei, RNA synthesis was stimulated, whereas neither zeatin nor ZBP alone had any effect. It has been shown that the activity of both RNA polymerase I and RNA polymerase II is stimulated, which implies that both ribosomal RNA and messenger RNA genes are increased.
Another approach to identify a possible cytokinin receptor was made by studying mutations in Arabidopsis in which the mutant calluses grow in a medium without cytokinin.
In these mutant calluses, a gene was identified which was designated CKI 1 (cytokinin independent 1). This gene encodes a 125 kd protein and this protein (CKI 1) which is localized in the plasma membrane may be a cytokinin-binding protein.
(v) Role of Calcium as a Second Messenger in Cytokinin Signal Transduction:
Calcium has been held as a second messenger in many signal transduction pathways in plants and animals. Calcium ions may also act as second messenger in cytokinin-induced responses. In such systems, Ca2+ acts through the regulatory protein, calmodulin (CaM), having four high affinity calcium binding sites.
Calmodulin itself is inactive as a regulator, but the CaM-Ca complex can bind and activate several enzymes designated CaM-regulated protein kinases. Another group of protein kinases known as Calcium-dependent protein kinases (CDPKs) have calmodulin as an integral part of their structure and their activity is influenced by calcium binding.
There is evidence that Ca2+ acts as a second messenger in cytokinin-induced bud formation in the moss Funaria hygrometrica.
The evidences are:
(a) When treated with cytokinin, there is simultaneous increase in calcium levels in filament cells and bud formation,
(b) When the growing medium is devoid of calcium, cytokinin is not able to induce bud formation,
(c) The requirement of Ca2+ in inducing bud formation can be substituted by a Ca2+ ionophore that facilitates the movement of ions across membrane in the presence of external calcium, and the passage of Ca2+ ions through Ca2+ channels is regulated by cytokinin.
(vi) Cytokinin and Auxin Regulate Plant Cell Cycle:
Both auxin and cytokinins are involved in the regulation of cell cycle by controlling the activity of cyclin-dependent kinases.
The cyclins are the regulatory subunits of enzymes like cyclin-dependent protein kinases (CDKs) which regulate the cell cycle in eukaryotes by means of protein phosphorylation. Auxin has been shown to regulate the expression of the gene that encodes CDC2 (cell division cycle 2) that is the major CDK. However, CDK alone is not sufficient to stimulate cell division.
In Arabidopsis tissue cultures, two G1 type cyclin proteins, viz., δ3 cyclin and δ2 cyclin have been identified. Cytokinin has been shown to stimulate the expression of the G1 cyclin gene that encodes δ3 cyclin protein, whereas sucrose, a carbon source of cultured tissues, stimulates the expression of the other G1 cyclin gene coding for the protein δ2.
Such observation suggests that the culture medium should contain a combination of auxin, cytokinins and a carbon source like sucrose that is necessary for the formation of active CDK-G1 cyclin complex. In such a culture, the cells of a dormant tissue may be induced to divide and enter the cell cycle through the action of CDK-cyclin complex which permits protein phosphorylation and cell cycle regulation.
