Read this article to learn about the production of haploid plants.
There are two approaches for the production of haploid plants. The two approaches are: (1) In Vivo Approach and (2) In Vitro Approach.
Haploid plants are characterized by possessing only a single set of chromosomes (gametophytic number of chromosomes i.e. n) in the sporophyte. This is in contrast to diploids which contain two sets (2n) of chromosomes. Haploid plants are of great significance for the production of homozygous lines (homozygous plants) and for the improvement of plants in plant breeding programmes.
Brief History:
The existence of haploids was discovered (as early as 1921) by Bergner in Datura stramonium. Plant breeders have been conducting extensive research to develop haploids. The Indian scientists Cuha and Maheswari (1964) reported the direct development of haploid embryos and plantlets from microspores of Datura innoxia by the cultures of excised anthers.
Subsequently, Bourgin and Hitsch (1967) obtained the first full-pledged haploid plants from Nicotiana tabacum. Thereafter, much progress has been made in the anther cultures of wheat, rice, maize, pepper and a wide range of economically important species.
Grouping of Haploids:
Haploids may be divided into two broad categories:
1. Monoploids (monohapioids):
These are the haploids that possess half the number of chromosomes from a diploid species e.g. maize, barley.
2. Polyhaploids:
The haploids possessing half the number of chromosomes from a polyploid species are regarded as polyhaploids e.g. wheat, potato. It may be noted that when the term haploid is generally used it applies to any plant originating from a sporophyte (2n) and containing half the number (n) of chromosomes.
In Vivo and in Vitro Approaches:
The importance of haploids in the field of plant breeding and genetics was realised long ago. Their practical application, however, has been restricted due to very a low frequency (< 0.001%) of their formation in nature.
The process of apomixis or parthenogenesis (development of embryo from an unfertilized egg) is responsible for the spontaneous natural production of haploids. Many attempts were made, both by in vivo and in vitro methods to develop haploids. The success was much higher by in vitro techniques.
In vivo techniques for haploid production:
There are several methods to induce haploid production in vivo.
Some of them are listed below:
1. Androgenesis:
Development of an egg cell containing male nucleus to a haploid is referred to as androgenesis. For a successful in vivo androgenesis, the egg nucleus has to be inactivated or eliminated before fertilization.
2. Gynogenesis:
An unfertilized egg can be manipulated (by delayed pollination) to develop into a haploid plant.
3. Distant hybridization:
Hybrids can be produced by elimination of one of the parental genomes as a result of distant (interspecific or inter-generic crosses) hybridization.
4. Irradiation effects:
Ultra violet rays or X-rays may be used to induce chromosomal breakage and their subsequent elimination to produce haploids.
5. Chemical treatment:
Certain chemicals (e.g., chloramphenicol, colchicine, nitrous oxide, maleic hydrazide) can induce chromosomal elimination in somatic cells which may result in haploids.
In vitro techniques for haploid production:
In the plant biotechnology programmes, haploid production is achieved by two methods.
1. Androgenesis:
Haploid production occurs through anther or pollen culture, and they are referred to as androgenic haploids.
2. Gynogenesis:
Ovary or ovule culture that results in the production of haploids, known as gynogenic haploids.
Contents
Androgenesis:
In androgenesis, the male gametophyte (microspore or immature pollen) produces haploid plant. The basic principle is to stop the development of pollen cell into a gamete (sex cell) and force it to develop into a haploid plant. There are two approaches in androgenesis— anther culture and pollen (microspore) culture. Young plants, grown under optimal conditions of light, temperature and humidity, are suitable for androgenesis.
Anther Culture:
The selected flower buds of young plants are surface-sterilized and anthers removed along with their filaments. The anthers are excised under aseptic conditions, and crushed in 1% acetocarmine to test the stage of pollen development.
If they are at the correct stage, each anther is gently separated (from the filament) and the intact anthers are inoculated on a nutrient medium. Injured anthers should not be used in cultures as they result in callusing of anther wall tissue.
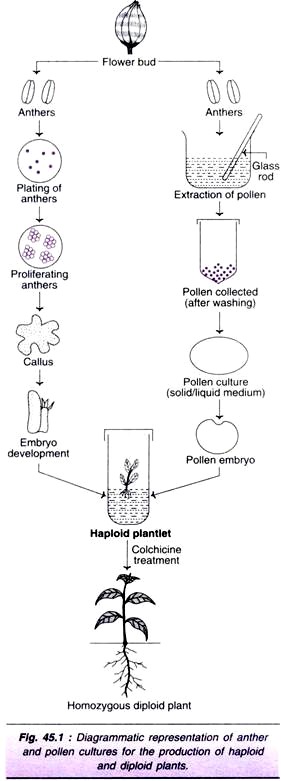
The anther cultures are maintained in alternating periods of light (12-18 hr.) and darkness (6-12 hrs.) at 28°C. As the anthers proliferate, they produce callus which later forms an embryo and then a haploid plant (Fig. 45.1).
Pollen (Microspore) Culture:
Haploid plants can be produced from immature pollen or microspores (male gametophytic cells). The pollen can be extracted by pressing and squeezing the anthers with a glass rod against the sides of a beaker. The pollen suspension is filtered to remove anther tissue debris.
Viable and large pollen (smaller pollen do not regenerate) are concentrated by filtration, washed and collected. These pollen are cultured on a solid or liquid medium. The callus/embryo formed is transferred to a suitable medium to finally produce a haploid plant (Fig. 45.1), and then a diploid plant (on colchicine treatment).
Comparison between anther and pollen cultures:
Anther culture is easy, quick and practicable. Anther walls act as conditioning factors and promote culture growth. Thus, anther cultures are reasonably efficient for haploid production. The major limitation is that the plants not only originate from pollen but also from other parts of anther. This results in the population of plants at different ploidy levels (diploids, aneuploids). The disadvantages associated with anther culture can be overcome by pollen culture.
Many workers prefer pollen culture, even though the degree of success is low, as it offers the following advantages:
i. Undesirable effects of anther wall and associated tissues can be avoided.
ii. Androgenesis, starting from a single cell, can be better regulated.
iii. Isolated microspores (pollen) are ideal for various genetic manipulations (transformation, mutagenesis).
iv. The yield of haploid plants is relatively higher.
Development of Androgenic Haploids:
The process of in vitro androgenesis for the ultimate production of haploid plants is depicted in Fig. 45.2.
The cultured microspores mainly follow four distinct pathways during the initial stages of in vitro androgenesis.
Pathway I:
The uninucleate microspore undergoes equal division to form two daughter cells of equal size e.g. Datura innoxia.
Pathway II:
In certain plants, the microspore divides unequally to give bigger vegetative cell and a smaller generative cell. It is the vegetative cell that undergoes further divisions to form callus or embryo. The generative cell, on the other hand, degenerates after one or two divisions—e.g., Nicotiana tabacum, Capsicum annuum.
Pathway III:
In this case, the microspore undergoes unequal division. The embryos are formed from the generative cell while the vegetative cell does not divide at all or undergoes limited number of divisions e.g. HyoScyamus niger.
Pathway IV:
The microspore divides unequally as in pathways I and II. However, in this case, both vegetative and generative cells can further divide and contribute to the development of haploid plant e.g. Datura metel, Atropa belladonna.
At the initial stages, the microspore may follow any one of the four pathways described above. As the cells divide, the pollen grain becomes multicellular and burst open. This multicellular mass may form a callus which later differentiates into a plant (through callus phase). Alternately, the multicellular mass may produce the plant through direct embryogenesis (Fig. 45.1).
Factors Affecting Androgenesis:
A good knowledge of the various factors that influence androgenesis will help to improve the production of androgenic haploids. Some of these factors are briefly described.
Genotype of donar plants:
The success of anther or pollen culture largely depends on the genotype of the donor plant. It is therefore important to select only highly responsive genotypes. Some workers choose a breeding approach for improvement of genotype before they are used in androgenesis.
Stage of microspore or pollen:
The selection of anthers at an ideal stage of microspore development is very critical for haploid production. In general, microspores ranging from tetrad to bi-nucleate stages are more responsive. Anthers at a very young stage (with microspore mother cells or tetrads) and late stage (with bi-nucleate microspores) are usually not suitable for androgenesis. However, for maximum production of androgenic haploids, the suitable stage of microspore development is dependent on the plant species, and has to be carefully selected.
Physiological status of a donar plant:
The plants grown under best natural environmental conditions (light, temperature, nutrition, CO2 etc.) with good anthers and healthy microspores are most suitable as donor plants. Flowers obtained from young plants, at the beginning of the flowering season are highly responsive. The use of pesticides should be avoided at least 3-4 weeks preceding sampling.
Pretreatment of anthers:
The basic principle of native androgenesis is to stop the conversion of pollen cell into a gamete, and force its development into a plant. This is in fact an abnormal pathway induced to achieve in vitro androgenesis. Appropriate treatment of anthers is required for good success of haploid production.
Treatment methods are variable and largely depend on the donor plant species:
1. Chemical treatment:
Certain chemicals are known to induce parthenogenesis e.g. 2-chloroethylphosphonic acid (ethrel). When plants are treated with ethreal, multinucleated pollens are produced. These pollens when cultured may form embryos.
2. Temperature influence:
In general, when the buds are treated with cold temperatures (3-6°C) for about 3 days, induction occurs to yield pollen embryos in some plants e.g. Datura, Nicotiana. Further, induction of androgenesis is better if anthers are stored at low temperature, prior to culture e.g. maize, rye. There are also reports that pretreatment of anthers of certain plants at higher temperatures (35°C) stimulates androgenesis e.g. some species of Brassica and Capsicum.
Effect of light:
In general, the production of haploids is better in light. There are however, certain plants which can grow well in both light and dark. Isolated pollen (not the anther) appears to be sensitive to light. Thus, low intensity of light promotes development of embryos in pollen cultures e.g. tobacco.
Effect of culture medium:
The success of another culture and androgenesis is also dependent on the composition of the medium. There is, however, no single medium suitable for anther cultures of all plant species. The commonly used media for anther cultures are MS, White’s, Nitsch and Nitsch, N6 and B5. These media in fact are the same as used in plant cell and tissue cultures. In recent years, some workers have developed specially designed media for anther cultures of cereals.
Sucrose, nitrate, ammonium salts, amino acids and minerals are essential for androgenesis. In some species, growth regulators — auxin and/or cytokinin are required for optimal growth. In certain plant species, addition of glutathione and ascorbic acid promotes androgenesis. When the anther culture medium is supplemented with activated charcoal, enhanced androgenesis is observed. It is believed that the activated charcoal removes the inhibitors from the medium and facilitates haploid formation.
Gynogenesis:
Haploid plants can be developed from ovary or ovule cultures. It is possible to trigger female gametophytes (megaspores) of angiosperms to develop into a sporophyte. The plants so produced are referred to as gynogenic haploids.
Gynogenic haploids were first developed by San Noem (1976) from the ovary cultures of Hordeum vulgare. This technique was later applied for raising haploid plants of rice, wheat, maize, sunflower, sugar beet and tobacco.
In vitro culture of un-pollinated ovaries (or ovules) is usually employed when the anther cultures give .unsatisfactory results for the production of haploid plants. The procedure for gynogenic haploid production is briefly described.
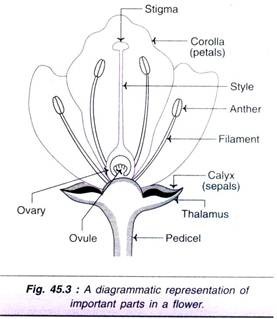
The flower buds are excised 24-48 hr. prior to anthesis from un-pollinated ovaries. After removal of calyx, corolla and stamens, the ovaries (see Fig. 45.3) are subjected to surface sterilization. The ovary, with a cut end at the distal part of pedicel, is inserted in the solid culture medium.
Whenever a liquid medium is used, the ovaries are placed on a filter paper or allowed to float over the medium with pedicel inserted through filter paper. The commonly used media are MS, White’s, N6 and Nitsch, supplemented growth factors. Production of gynogenic haploids is particularly useful in plants with male sterile genotype. For such plant species, this technique is superior to another culture technique.
Limitations of Gynogenesis:
In practice, production of haploid plants by ovary/ ovule cultures is not used as frequently as anther/ pollen cultures in crop improvement programmes.
The major limitations of gynogenesis are listed:
1. The dissection of unfertilized ovaries and ovules is rather difficult.
2. The presence of only one ovary per flower is another disadvantage. In contrast, there are a large number of microspores in one another.
However, the future of gynogenesis may be more promising with improved and refined methods.
Identification of Haploids:
Two approaches based on morphology and genetics are commonly used to detect or identify haploids.
Morphological Approach:
The vegetative and floral parts and the cell sizes of haploid plants are relatively reduced when compared to diploid plants. By this way haploids can be detected in a population of diploids. Morphological approach, however, is not as effective as genetic approach.
Genetic Approach:
Genetic markers are widely used for the specific identification of haploids. Several markers are in use.
i. ‘a1‘ marker for brown coloured aleurone.
ii. ‘A’ marker for purple colour.
iii. ‘Lg’ marker for ligule less character.
The above markers have been used for the development of haploids of maize. It may be noted that for the detection of androgenic haploids, the dominant gene marker should be present in the female plant.
Diploidizatioim of Haploid Plants (Production of Homozygous Plants):
Haploid plants are obtained either by androgenesis or gynogenesis. These plants may grow up to a flowering stage, but viable gametes cannot be formed due to lack of one set of homologous chromosomes. Consequently, there is no seed formation.
Haploids can be diploidized (by duplication of chromosomes) to produce homozygous plants. There are mainly two approaches for diploidization— colchicine treatment and endomitosis.
Colchicine Treatment:
Colchicine is very widely used for diploidization of homologous chromosomes. It acts as an inhibitor of spindle formation during mitosis and induces chromosome duplication. There are many ways of colchicine treatment to achieve diploidization for production of homozygous plants.
1. When the plants are mature, colchicine in the form of a paste is applied to the axils of leaves. Now, the main axis is decapitated. This stimulates the axillary buds to grow into diploid and fertile branches.
2. The young plantlets are directly treated with colchicine solution, washed thoroughly and replanted. This results in homozygous plants.
3. The axillary buds can be repeatedly treated with colchicine cotton wool for about 2-3 weeks.
Endomitosis:
Endomitosis is the phenomenon of doubling the number of chromosomes without division of the nucleus. The haploid cells, in general, are unstable in culture with a tendency to undergo endomitosis. This property of haploid cells is exploited for diploidization to produce homozygous plants.
The procedure involves growing a small segment of haploid plant stem in a suitable medium supplemented with growth regulators (auxin and cytokinin). This induces callus formation followed by differentiation. During the growth of callus, chromosomal doubling occurs by endomitosis. This results in the production of diploid homozygous cells and ultimately plants.