Let us make an in-depth study of the definition, principle, protocol and importance of the cell suspension culture.
Definition:
Suspension culture is a type of culture in which single cells or small aggregates of cells multiply while suspended in agitated liquid medium. It is also referred to as cell culture or cell suspension culture.
Principle:
Callus proliferates as an unorganised mass of cells. So it is very difficult to follow many cellular events during its growth and developmental phases. To overcome such limitations of callus culture, the cultivation of free cells as well as small cell aggregates in a chemically defined liquid medium as a suspension was initiated to study the morphological and biochemical changes during their growth and developmental phases.
To achieve an ideal cell suspension, most commonly a friable callus is transferred to agitated liquid medium where it breaks up and readily disperses. After eliminating the large callus pieces, only single cells and small cell aggregates are again transferred to fresh medium and after two or three weeks a suspension of actively growing cells is produced.
This suspension can then be propagated by regular sub-culture of an aliquot to fresh medium. Ideally suspension culture should consist of only single cells which are physiologically and biochemically uniform. Although this ideal culture has yet to be achieved, but it can be achieved if it is possible to synchronize the process of cell division, enlargement and differentiation within the cell population.
The culture of single cells and cell aggregates in moving liquid medium can be handled as the culture of microbes. The suspension culture eliminates many of the disadvantages ascribed to the callus culture on agar medium. Movement of the cells in relation to nutrient medium facilitates gaseous exchange, removes any polarity of the cells due to gravity and eliminates the nutrient gradients within the medium and at the surface of the cells.
Protocol:
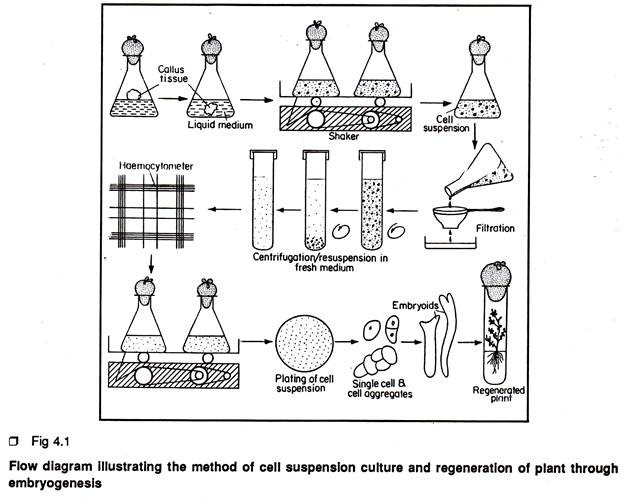
1. Take 150/250 ml conical flask containing autoclaved 40/60 ml liquid medium (Fig 4.1).
2. Transfer 3-4 pieces of pre-established callus tissue (approx. wt. 1 gm. each) from the culture tube using the spoon headed spatula to conical flasks.
3. Flame the neck of conical flask, close the mouth of the flask with a piece of alluminium foil or a cotton plug. Cover the closure with a piece of brown paper.
4. Place the flasks within the clamps of a rotary shaker moving at the 80-120 rpm (revolution per minute)
5. After 7 days, pour the contents of each flask through the sterilized sieve pore diameter -60µ- 100µ and collect the filtrate in a big sterilized container. The filtrate contains only free cells and cell aggregates.
6. Allow the filtrate to settle for 10-15 min. or centrifuge the filtrate at 500 to 1,000 rpm and finally pour off the supernatant.
7. Re-suspend the residue cells in a requisite volume of fresh liquid medium and dispense the cell suspension equally in several sterilized flasks (150/250 ml). Place the flasks on shaker and allow the free cells and cell aggregates to grow.
8. At the next subculture, repeat the previous steps but take only one-fifth of the residual cells as the inoculum and dispense equally in flasks and again place them on shaker.
9. After 3-4 subcultures, transfer 10 ml of cell suspension from each flask into new flask containing 30 ml fresh liquid medium.
10. To prepare a growth curve of cells in suspension, transfer a definite number of cells measured accurately by a haemocytometer to a definite volume of liquid medium and incubates on shaker. Pipette out very little aliquot of cell suspension at short intervals of time (1 or 2 days interval) and count the cell number. Plot the cell count data of a passage on a graph paper and the curve will indicate the growth pattern of suspension culture.
Importance of Cell Suspension Culture:
The culture of single cells and small aggregates in moving liquid medium is an important experimental technique for a lot of studies that are not correctly possible to do from the callus culture. Such a system is capable of contributing many significant information’s about cell physiology, biochemistry, metabolic events at the level of individual cells and small cell aggregates.
It is also important to build up an understanding of an organ formation or embryoid formation starting from single cell or small cell aggregates. The technique of plating out cell suspension on agar plates is of particular value where attempts are being made to obtain single cell clones.
Suspension culture derived from medicinally important plants can be studied for the production of secondary metabolites such as alkaloids and a considerable amount of industrial effort is being placed on the exploitation and expansion of this area.
Mutagenesis studies may be facilitated by the use of cell suspension cultures to produce mutant cell clones from which mutant plants can be raised. Cell population in a suspension can be subjected to a range of mutagenic chemicals e.g. ethyl methane-sulphonate (EMS), N-nitroso-N- methyl urea etc.
The mutagens can be added directly in the liquid medium. After the mutagen treatment, cells are plated on agar medium for the selection of mutant cell clones. The hope is that permanent changes in the DNA patterns of some of the cells would be achieved by such treatments.
Plants could be raised from the mutant cell clones and the mutant plants are selected from the population either by morphological differences or by metabolic/biochemical differences. The selected plants can then be grown on and propagated further to produce a mutant population for evaluation studies.