In this article we will discuss about:- 1. Meaning of Stomata 2. Types of Stomata 3. Distribution 4. Daily Periodicity 5. Diffusive Capacity 6. Dynamic 7. Mechanism 8. Aspects of the Physiology of Stomatal Opening.
Contents:
- Meaning of Stomata
- Types of Stomata
- Distribution of Stomata
- Daily Periodicity of Stomatal Movements
- Diffusive Capacity of Stomata
- Dynamic of Stomata
- Mechanism of Stomatal
- Aspects of the Physiology of Stomatal Opening
1. Meaning of Stomata:
The epidermal surface of a leaf has several tiny pores called stomata which are microscopic and are surrounded by two guard cells which control their opening and closing. Cell wall of the guard cells adjacent to the stomatal pore is thicker and more inelastic than the wall adjacent to the surrounding epidermal cells. An increase in turgor pressure will cause the more elastic part of the guard cell wall to stretch considerably (Fig. 8-4).
The cell wall bordering the stomatal pore is thicker than that next to the surrounding cells. The guard cells contain chloroplast, while the inelastic thicker part of the wall encloses the pore. When the water is lost, the guard cells decrease in volume; their walls straighten and the aperture is closed.
In some plants, the guard cells are accompanied by epidermal cells which are different from the rest of the epidermal cells. These cells are called subsidiary or accessory cells. The guard cells possess chloroplasts but the eidermal cells lack them. Photosynthesis in the guard cells is at a reduced rate.
2. Types of Stomata:
Morphologically, four types of stomata are distinguished in the dicots and this classification is based on the arrangement of the epidermal cells adjacent to the guard cells.
i. Anomocytic type:
These are also called ranunculaceous types. In this type the guard cells are surrounded by some cells which do not differ from the other epidermal cells.
ii. Anisocytic type:
These are also called cruciferous types. In this type the guard cells are surrounded by three unequally sized subsidiary cells.
iii. Paracytic type:
This is also called rubiaceous type. Here each guard cell is accompanied by one or more subsidiary cells which are parallel to the long axis of the epidermal cell.
iv. Diacytic type:
This is also called caryophyllaceous type. Here the stoma is surrounded by two subsidiary cells.
v. Actinocytic type:
In this type the stomata are surrounded by a circle of radiating cells.
In the monocots as many as 4 types are distinguished on the basis of number of subsidiary cells surrounding the guard cells.
Some of the recent workers have distinguished as many as 15 main types of stomata in ferns, gymnosperms and angiosperms and for details reference may be made to any textbook on plant anatomy.
3. Distribution of Stomata:
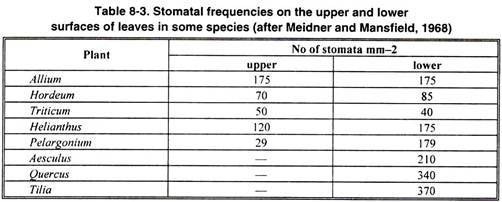
Stomata are more frequent on the lower surface of the leaves but their occurrence on both the surfaces has also been commonly noted.
They are distributed only on the lower surface e.g. in oak, apple and orange; more on lower surface than on the upper surface e.g. in bean, maize, and sunflower and equally on both the surfaces e.g., in maize, oats; only on the upper surface e.g. water lily and absent or functionless e.g. in submerged water plants.
When fully opened, the stomatal pore has a width of 3 to 12 µ and a length of 10 to 40 µ. The number of stomata range from 100 to 60,000 per square centimetre.
The number of stomata per unit of leaf is called stomatal frequency. Stomatal index is defined as the percentage number of stomata as compared to all the epidermal cells in a unit area of leaf [I = S/(E+S)× 100 ]. The total pore area, when stomata are fully opened, is 1 to 2% of the total leaf surface.
4. Daily Periodicity of Stomatal Movements:
A number of environmental factors such as solar radiation, air and soil temperatures, wind velocity and atmospheric humidity exhibit daily periodicities. The stomata of all plants also show daily periodicities of opening and closing, as their behaviour depends upon these climatic factors.
On a ‘representative summer day’ in the moist temperate zone, when the sky is cloudless, a soil has favourable water supply i.e., at field capacity, and a maximum temperature is in the range of 30—35°C.
While the light, soil and air temperature, wind velocity and atmospheric humidity show regular daily periodicity, the stomata of most mesophytic plants are open during the entire or most of the day light period and close at night. Their maximum diffusive capacity being attained during mid-day.
The stomata open at dawn under the influence of light factor and maximum opening of stomato occurs in less than an hour. Under standard conditions the water contents and turgor of the leaf cells decrease progressively during the day and as a result stomata begin to close during the mid-day and completely close before sunset.
At this afternoon hour the effect of water factor is predomint over the light factor when the internal water deficit develops in the leaves somewhat earlier in the day than under standard day conditions, stomata partially close during midday. This results in the decrease in transpiration.
The resultant decrease in transpiration permits an increase in water content of the leaves and for a while stomata widen again and transpiration attains a secondary maxima during the early afternoon. Subsequently, the water deficit of the leaf increases again and stomata close again by the sunset.
When the sky is overcast and temperature is low and soil well watered, the stomata of most species are not so wide open as in bright light and do not remain open as long as under standard conditions.
Light factor is ineffective in inducing stomatal opening. In very dry weather they open incompletely in the morning and soon close again before noon as a result of loss of water by the leaf. Under conditions of maximum heat and dryness, they remain closed whole day long and night and open only for a short time during the early morning hours.
The behaviour of stomata also varies with different plant species as given below:
In potato, cabbage, beet, etc. under optimum conditions of water supply stomata tend to remain open during day and night. The nocturnal opening is favoured by high temperatures and reduced partial pressure of oxygen in the intercellular spaces of leaf.
In cereals e.g., maize and rice, stomata always shut at night, moreover they close very early in the evening and at the slightest deficiency of moisture, they may close even in the morning hours. In alfa- alfa, they open during day and close at night.
5. Diffusive Capacity of Stomata:
The stomatal pores allow the exchange of water vapours between the external environment and the interior of the leaf. When fully opened, the combined pore area consists of 1 -2% of the total leaf area but the diffusion of water vapor through the pores often exceeds 50% of that evaporating from a free water surface of the same area.
That is, water vapors pass out of a small opening 5-25 times as fast as from an equal area of free water surface. Brown and Escombe reported that diffusion through small circular pores is more and were nearly proportional to the perimeter or diameter of the pore than to its area.
The reason for more rapid diffusion of water vapor through small pores per unit area than diffusion from large free water surface is that in both cases water vapor becomes increasingly concentrated over the evaporating surface, decreasing the (Fig. 8-3) vapor pressure gradient.
This, in turn, decreases the rate of diffusion. However, over a large free surface of water, water vapor would collect and form several overlapping diffusion “shells” or “caps”. The result is a heavy blanket of water vapor which covers the water surface, lowering the vapor pressure gradient over the whole area.
The only appreciable diffusion that will take place will be around the perimeter where the least resistance from water vapor in the air will be encountered. The magnitude of this “perimeter diffusion” when compared with diffusion from the complete surface is relatively insignificant because of low perimeter/area ratio.
Water vapor diffusing through a small isolated poor will also form a diffusion shell and lower the vapor pressure gradient. However, in this case, “perimeter diffusion” is much more significant because of high P/A ratio; perimeter of an enclosed surface becomes larger in relation to the area of surface as that surface becomes smaller.
Therefore, the smaller a pore, more nearly diffusion through it becomes proportional to its perimeter. Suppose, the water surface is covered by a membrane containing numerous small pores which are placed far apart so that their diffusion shells do not interfere with one another; it is conceivable under these conditions, that the perimeter diffusion will be very high.
This situation is similar to that of water diffusion through the stomata of a leaf. Stomata, when open, do not present a barrier to the diffusion of water vapor from the interior of the leaf into the atmosphere. Thus, the efficiency of diffusion of water vapor through the stomatal pore is very high.
6. Dynamic of Stomata:
Guard cells of stomata are smaller than the other epidermal cells. This attribute helps the guard cells to undergo quick turgor changes. Guard cells invariably contain chloroplasts which are capable of performing photosynthetic light reactions.
However, the scale of photosynthesis is very low. Red and blue parts of light affect the process significantly. In addition guard cells are also shown to contain microbodies which participate in glycolate metabolism.
Guard cells chloroplasts have peripheral reticulum which resembles the mesophyll cells of the C4 plants. In this respect the two systems have PEP carboxylation and transport malate to the outside. Guard cell chloroplasts are also rich in starch and related polysaccharides (e.g., in Phaseolus and Allium species).
The walls of the guard cells are differently thickened on the outer and the inner sides. Plamodesmata connect the guard cell with the adjacent subsidiary or epidermal cells. Indeed the adjacent subsidiary or the epidermal cells store inorganic salts including K+. Usually stomata remain open during the day time and close at night.
However, in many succulents stomata remain open even at night stomata can also open in responses to high temperature, high pH and low levels of CO2. Under the conditions which are reverse of the above mentioned ones, they close. It is also observed that osmotic potential of the about- to-open stomata, guard cells is 30-45 atm.
Increased osmotic potential causes an increase in the turgidity of guard cells. As a result volume of guard cell increases and guard cells bulge out. Consequently the pore is opened. Two phases are recognized in stomtal opening and these are tension phase and motor phase. In the former, the guard cells increase in breadth while in the latter outward bending is caused.
Several phytohormones and phenolic compounds affect stomatal opening and closing. Thus, cytokinins, cyclic AMP promote stomatal opening while abscises acid and phenolic compounds like salicylic acid reduce stomatal aperture. Further, when the stomata bearing epidermal peels are immersed in Na+ and K+ ions, stomatal pores open. On the other hand, Ca2+ ions close the aperture.
There has been the suggestion that cytokinins possibly promote K+ ions influx in the guard cells and same is done by the cAMP. In general five different types of stomatal movement are recognized.
Photo-active movements:
In this category of stomata light controls their movement directly or indirectly. The movement of guard cells may be due to the hydrolysis of starch in the guard cell or photosynthesis by the guard cells chloroplasts or due to the reduced concentrations of the CO2. In addition blue light and increased pH also affect the opening.
Scoto-active movements:
These types are encountered in succulents. Here stomata remain closed during the day time but are opened at night. The movement may be carried out through organic acid metabolism.
Hydro-active movement:
Stomata are opened if the epidermal cells lose more water. The turgid conditions of the epidermal cells close the stomata. This usually happens during mid-day.
Autonomous movement:
Some guard cells display diurnal or rhythmic pulsation at a rate of about 10-15 minutes. Some workers regard such movements as autonomous while others consider that such rhythmic pulsations are caused by water content, starch-sugar interconversion or concentrations of CO2.
Passive and active movements:
Some investigators have proposed that opening of stomata is an active state and is caused by the turgor changes in the guard cells.
On the other hand, the passive movement comprises the closure of stomata. This is caused by turgor changes in the epidermal or subsidiary cells. Some workers regard that the closure of stomata is an active process.
7. Mechanism of Stomatal:
(i) Starch-sugar hypothesis:
Lloyd, Doftified and Sayre observed that starch content of guard cells is high in the dark and low in light.
This is unlike the other epidermal cells and mesophyll cells where opposite effect is observed. It is also noted that a high pH favours opening and a low pH the closure of stomata.
In light CO2 concentration of guard cells and neighbouring cells is lowered being consumed in photosynthesis; hence the rise in pH.
In the dark, CO2 of respiration accumulates in the guard cells; hence there is fall in pH of the guard cells.
A high pH favours conversion of starch into osmotically active reducing sugars, resulting in an increase of turgor in the guard cells and stomata open. The reverse reaction occurs when pH is lowered.
The enzyme phosphorylase, present in the chloroplasts catalyzes this reaction in the presence of inorganic phosphate as follows:
Steward (1964) criticized the above scheme and pointed out that unless glucose 1-phosphate was further converted to glucose and inorganic phosphate, no appreciable change in osmotic potential can be obtained.
Inorganic phosphate on the left side of the above equation is just as osmotically active as glucose 1 -phosphate on the right.
At high pH starch is converted to glucose in the following way to make the stomata open:
The closing of stomata requires metabolic energy in the form of ATP. With the help of ATP and in the presence of oxygen, the enzyme hexokinase converts glucose to glucose 1 -phosphate. The latter is then converted to starch.
There are several drawbacks of the starch hydrolysis theory. For instance, the increase of the osmotic potential of the guard cells during stomatal opening cannot be explained by the formation of glucose from starch in its entirety.
It also does not account for the extra-effectiveness of blue light during the opening of stomata. In some plants there is no starch present in the guard cells (e.g., Allium sp.).
Inter-conversion of starch-sugar is very slow and does not satisfactorily explain the quick stomatal movement or responses to water levels.
Main objectives against the theory:
i. Many stomatal cells do not have starch ⇋ glucose converssion e.g., onion has fructason.
ii. Many monocots do not have starch in guard cell.
iii. Stomata open more rapidly in light.
(ii) Glycolic acid hypothesis:
Zalitch (1963) has proposed that glycolic acid plays an important role in stomatal opening. He has suggested the formation of carbohydrates from glycolic acid, thus increasing the osmotic value.
The general assumption is that ATP produced in this mechanism furnishes the required energy needed for the opening and closing of stomata.
It is believed that energy possibly participates in the active pumping of water into the guard cells.
The main objection against this theory is the speculative nature of the role of energy in ‘pumping’ of water.
(iii) Several recent studies have demonstrated the occurrence of ATP and glycolate in the guard cells through photosynthesis. Studies of Rama Das and Raghvendra (1976) using inhibitors of Photosystem 1 and Photosystem II, have provided unequivocal support to this view point.
Their studies have further revealed that in vivo the process of photophosphorylation possibly satisfies the energy requirements for stomatal movements. Thus, the dependency of light for the opening of stomata lay in the energy matabolism rather than in carbon metabolism.
Using isolated epidermal strips of Commelinabengalensis it was shown that the opening of stomata in light was under the control of cyclic photophosphorylation. When the inhibitors of cyclic photophosphorylation were added to the epidermal strips, stomata closed rapidly while the inhibitors of non-cyclic photophosphorylation did not influence the stomatal aperture.
Similarly the catalysts of the cyclic and not the noncyclic photophosphorylation promoted stomatal opening. Further the involvement of ATP in the enhancement of stomatal opening in the presence of K+ was also confirmed through the addition of the inhibitors like, 2, 4-dinitrophenol which caused the closure of the stomata.
On the other hand, the addition of HCO3− ions in the medium caused the closure of stomata. These authors also speculated a mechanochemical system operating in the stomatal movement, thus explaining the requirement of energy.
(iv) Adenosine triphosphate (ATP) is also produced during respiration for the dark opening of stomata. Recent studies have also demonstrated that stomatal movement was also regulated by the hormones. For instance, the stomata were opened by the cytokinins either directly or by functioning as metabolic sinks.
On the other hand, several inhibitors like ABA caused their closure. It may also be mentioned that ABA functions in the presence of CO2 only. In fact, ABA was produced in the leaf cells under stress conditions and antagonises the cytokinin action. This inhibitor is also shown to change the diffusion and permeability of the guard cells. In this way K+ influx is inhibited.
Thus, K+ from guard cells passes into the epidermal or subsidiary cells and the guard cells collapse resulting in the closure of the stomata. In this efflux of H+ ions is also avoided. In the guard cells the acidity is raised or pH becomes acidic or low. Similar situation prevails with excessive CO2. It is well accepted that low pH and excessive organic acids stimulate starch synthesis.
Raschke (1979) has proposed a scheme based on interaction of the feedback and feed-forward pathways (see above) (Fig. 8-6). According to him stomatal movements can be considered manifestations of a central system optimizing gas exchange and employing feedback and feed forward methods of control.
(v) Modern concept based on K+ influx:
Guard cells dispersed in the epidermal tissue synthesize malate which contributes to the enhancement of turgor needed for their opening. Guard cells are supposedly heterotrophic since their primary supply of reduced carbon is achieved through photosynthesis within the leaf.
Apparently, metabolism of C4 acids in such cells is not directly associated with the Reductive pentose phosphate pathway. A large body of evidences has shown that the increase in solutes of the guard cells during stomatal opening is attributed to the enhancement of potassium ions concentration besides chloride and malate.
The precise amount of malic acid and chloride, of course, varies with the species, as the counter ions to potassium. Osmotic changes within the guard cells regulate the stomatal movement. Thus with an increase in the solute concentration within guard cells water moves in from the adjacent cells resulting in increased turgor in the guard cells.
This increase is relative to the adjacent cells and there is opening of stomata. The guard cells because of unequal thickness of the two walls bulge out like a blown up balloon. On the contrary, in grasses, increase in osmotic pressure of the guard cells causes the expansion of ends of cells resulting in stomatal opening.
Guard cells chloroplasts contain starch during the day and are degraded at night. Nothing precisely can be said regarding the availability of malate in the stomatal opening though degradation of starch during the day possibly provides PEP as a precursor for malate formation via PEPc and MDH. The situation may be compared with malate metabolism during the dark phase of CAM.
Recent evidences have shown the presence of PEPc in the guard cells and malic acid synthesized via this enzyme would provide protons for counter exchange with K+ arriving from the adjacent subsidiary cells Fig. 8-5. Under night or dark conditions, the C4 acid possibly decarboxylates and the C3 precursors are metabolised back to starch.
However, it may be noted that CO2 released is not resimilated via RPP pathway as is the case in CAM or C4 plants. Mitochondrial respiration possibly provides the required energy for the conversion of pyruvate to starch.
In some of the cases well studied it seems that malate and aspartate are the primary initial products of CO2 fixation. There is also some thinking that guard cell chloroplasts lack or have very little RuBPc activity. They may be primarily concerned with the supply of energy rather than RPP pathway concerned with carbon assimilation.
Mansfield (1990) has proposed that ABA-induced increases in cytosolic free calcium ions (Ca2+cyt) triggers intercellular processes resulting in closure of stomata. Some evidences point out the role of calmodulin (Ca2+ -binding protein) in Ca2+ mediated reactions.
Cyclic AMP, the second messenger, has been suggested to play some role in stomatal mechanism; it may remove inhibitory effect of Ca2+ and ABA in guard cells since it enhances stomatal opening. On the contrary the degradation of CAMP would induce closure of stomata.
Osmotica in Guard Cells:
It is generally accepted that opening of stomata was the osmotic effect of an accumulation of K+ salt and also in some plants Na+ in the guard cells. K+ has been discovered in guard cells of open stomata of several plant species including CAM plants where stomata open in the night. Sodium has been found in addition to K+ in some species growing under saline conditions.
The accumulation and discharge of K+ contents account for the turgor changes in guard cells that give rise to stomatal movements. Further K+ malate (and salts of other acids of the TCA cycle) or KCl appear to be dominating osmotica in guard cells of open stomata: which salt dominates is dependent upon the species. Participation of Cl− in stomatal movement has also been demonstrated in some species.
It has been suggested that guard cells obtain K+ and CI–through the apoplast. However, malate can be produced within the guard cells. Subsidiary cells appear to be the effective reservoirs of ions. During stomatal movements, there are differences between the K+ and CI– distribution of guard and subsidiary cells.
The presumed function of the stomata is related to the metabolic activity of the mesophyll; its assimilation of CO2 and its water relations. In succulent plants, most of the stomata are completely closed during the day time. It is a device to reduce transpiration.
At the same time, CO2 uptake for photosynthesis is also lowered. In Bryophyllum, Sedum etc. during the day time the pH of the cell sap is low and is highest in the evening. These fluctuations were chiefly due to the malate. The change in acid content is called diurnal acid cycle. Using 14CO2 (isotopic), it is observed that malate gets fixed during the night. Sometimes this is called dark fixation of CO2.
The formation of malate occurs as follows:
The PEP is derived from starch formed during photosynthesis during the day. In the succulents high respiration releases CO2 during the night, which is stored as malate? During the day photosynthesis occurs and stomata close due to heat and CO2 is obtained from malate even in the closed stomata. This is an excellent example of adaptation in the succulents.
The metabolic reactions involved in the opening and closing of stomata may be summarized as under:
Thus, according to Sayre and Steward (1964) there is a close correlation between light, the photosynthetic lowering of CO2– concentration and stomatal opening.
There is some evidence that light has direct effect on the stomata through a pathway independent of CO2. Perhaps the light energy is converted into chemical energy, which is then used to move ions from surrounding cells into the guard cells.
This increase in ions concentration would cause water to move in osmotically. Many other more complex explanations have been offered. According to Bowling (1976), who gave the malate switch hypothesis of stomatal movement the change in the pH of the guard cells creates a gradient for monovalent malate towards either guard cells or epidermal cells causing a diffusion of K+ (bound to monovalent malate) along with the gradient.
It has been observed that epidermal tissues produce appreciable quantities of malate and the changes in malate levels are closely and positively correlated with the stomatal aperture. Further, it is known for certain that epidermal tissue contains highly active enzymes not only for malate synthesis but also for malate degradation.
It is therefore believed that malate production and metabolism could occur in the guard cells and was also supported by the auto-radiographic studies. It is also assumed that guard cells can derive malate through phosphoenolpyruvate carboxylation.
The Mechanism of Mid-day Stomatal Closure:
This aspect is not fully understood to date though several hypotheses have been advanced to provide suitable explanations.
These are:
I. Due to the inability of the plant to absorb and replace transpired water with same rapidity.
II. Due to an accumulation of CO2 in leaf intercellular spaces. The accumulation of CO2 is caused due to high cellular respiration and a decrease in photosynthesis due to high temperature.
III. Due to a direct effect of a decrease in atmospheric humidity adjacent to a leaf affecting the elasticity of guard cell walls.
Mid-day closure of stomata last for one to several hours and leads to an increase in leaf temperature causing additional loss of water vapours from the leaf. Consequently there is transient wilting of the leaf.
Summary of the Mechanism of Guard Cell Movement:
The driving force causing stomatal opening is called osmotic uptake of water by the guard cells and hence increase in hydrostatic pressure. This results in deformation of the opposing cells that increase the size of the opening between them.
Stomatal closure follows a loss of water and the resulting decrease in hydrostatic pressure and relaxation of guard cell walls. Over the years several mechanisms have been proposed to explain alerting osmotic concentrations of guard cells.
Most of these view-points have taken the distribution of chloroplasts in guard cells in view, Several traditional theories tended to take into account sugar or other ions contributing to the osmotic changes in guard cells.
The number of chloroplasts per guard cell varies and in some species chloroplasts are absent. Further the level of Rubisco in the guard cells is weak and thus photosynthesis in guard cells is very low. In the late 1960s it was demonstrated that level of K+ in guard cells was high in open and low in closed stomata.
Thus an accumulation of K+ in guard cells is now well accepted as a universal process in opening stomata. The amount of K+ determines the size of stomatal opening and is regulating the osmotic potential of guard cells.
Figure 8-7 is a simplified version for ion flow linked with guard cells during stomatal opening. K+ uptake is driven by an ATP ase-proton pamp in the plasma membrane. Ions accumulation in the vacuole decreases the water potential of the guard cells. This results in the increase in the osmotic uptake of water and enhanced turgor.
ATP-powered proton pump located on the plasma membrane stimulates ion uptake and is supported by two evidences. First, fusicoccinstimulates both stomatal opening and extrusion of active proton pump. Second, vanadate inhibits the proton pump also inhibits stomatal opening. It is believed that extrusion of proton tends to hyperpolarize the plasma membrane.
This then permits the uptake of K+ in response to the potential difference or charge gradient across the membrane. Figure 8.also shows entry of CI” ions which balances the charge of K+ ions. K+ uptake is also balanced by another organic ion, malate which is generated within the guard cells through PEPc system. The role of CI– or malate ions or both in acting as counterion is dependent on species.
Malate so contributes towards maintaining the pH during solute accumulation. Thus, proton loss will increase cellular pH; the synthesis of malate (as shown) will replenish the supply of proton lost by extrusion and hence help in maintaining the normal levels of pH.
The data on malate levels support the above contention when estimated in opened or closed stomata. Guard cells have high activity of PEPc and also there is decrease in the starch content of open stomata, Factors which affect stomatal opening also cause malate concentrations and in turn affects PEPc activity.
In fact fusicoccin stimulates stomatal opening and also enhances the activity of PEPc. ABA induces stomatal closure and reduces PEPc activity. In summary, both K+ and CI– and malate accumulation in the vacuole of the guard cells lowers the osmotic potential and the water potential of the guard cells.
Consequently there is uptake of water by the guard cells causing the stomate to open. Closure of the stomata possibly follows the events which are reverse of the opening. It has been observed that closure is much faster than opening suggesting the existence of other metabolic pumps causing active efflux of ions.
One view is that signals for closure of the stomata stimulate Ca2+ uptake by the guard cells cytosol resulting in depolarization of the membrane resulting in opening of channels to extrude malate-2 and CI–. The extrusion takes place into the adjacent subsidiary and epidermal cells.
Available evidences point towards the non-cyclic photophosphorylation or mitochondrial respiration and oxidative phosphorylation contributing to the ATP generation. Guard cells have been shown to contain large number of mitochondria. Respiratory inhibitors such as azide, cyanide and 2, 4-dinitrophenol inhibit stomatal opening.
8. Aspects of the Physiology of Stomatal Opening:
I. Stomatal opening dependent on CO2 concentration:
Sufficient evidence is available regarding stomatal response to changes in CO2 concentration in the atmosphere. When intensity of light is sufficient, concentration of CO2 is lowered by active photosynthesis in the leaf mosophyll and also guard cells.
Starch thus produced is hydrolysed and consequently stomata open. On the contrary, stomata close in darkness or under low intensity of light, when CO2 evolved from respiration is accumulated and photosynthesis is precluded. Stomatal response to several other environmental factors like water stress, temperature could also be explained in terms of their sensitivity to CO2 concentrations.
The closure of stomata during mid-day is attributed to increase in temperature. In some species it causes increase in CO2 concentration. When the leaves are in a state of water stress, the sensitivity of stomata to CO2 also increases.
II. Stomatal opening not associated with CO2 concentration:
Light induced opening:
In sweet potato some of the etiolated leaves lack chlorophyll and hence photosynthesis. In these leaves CO2 concentration is not lowered in light. Of the different light spectra, blue light was most efficient in causing stomatal opening. The results are interpreted to mean that blue light either did not permit conversion of sugars into starch or accelerated hydrolysis of starch. Apparently, CO2-independent opening effect of blue-light was similar in action to the removal of CO2 in causing hydrolysis of starch.
Temperature-induced opening:
In some species, when the temperature increases it causes stomatal closure by increasing CO2 concentration within the leaf. In some species stomata open in darkness when the temperature increases. It has been observed that the relative importance of CO2– induced closing and temperature-induced opening differ in different species. In onion, guard cells do not contain starch, and in them the opening is enzyme controlled hydrolysis of starch.Under light, stomata open either by CO2 removal or due to blue light, both causing hydrlysis of starch.