In this article we will discuss about the structure of pistil, explained with the help of suitable diagram.
The pistil is the female part of the flower, which is divisible into stigma, style, and ovary.
It is adapted to receive the pollen, aid its germination, and subsequently entry of the pollen tube into the ovary, ovule and finally into the embryo sac for fertilization.
a. The Stigma:
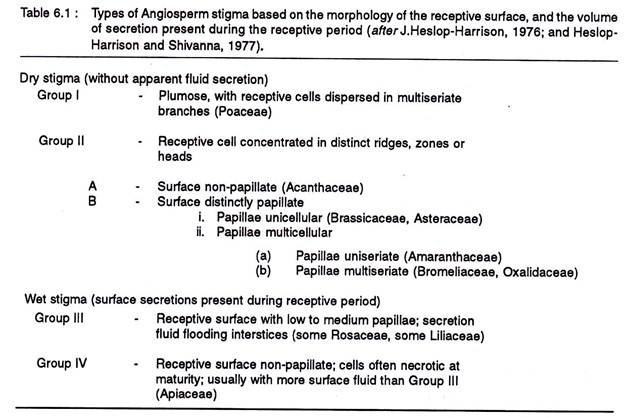
The stigma is the specialized part of the pistil on which the pollen grains are trapped during pollination. There is considerable variation in the nature of the stigma, and accordingly it may be of the wet or the dry type.
These two basic types based on the amount of secretion present during the receptive period, decoration of the receptive surface with glandular cells or papillae, and anatomical details of the papillae, have been further classified into four groups-I, II, III, IV. (Table 6.1).
From a broader perspective the composition of the secretion produced by the subepidermal cells of the stigma are highly heterogenous and include lipids, proteins, and glycoproteins, different carbohydrates, amino acids, arabionogalactans, Ca++ and phenols.
A range of enzymes has also been located, where the non-specific esterases are predominant. Cytochemical demonstration of nonspecific esterases has become a standard method of localization of the receptive surface of the stigma.
In its early development, the stigmatic epidermis is covered with a cuticle, whereas in some plants the cuticle has an extracellular proteinaceous li- poidal layer, called pellicle. This layer helps in the capture and hydration of pollen grains and also serves as a recognition site during pollen pistil interactions.
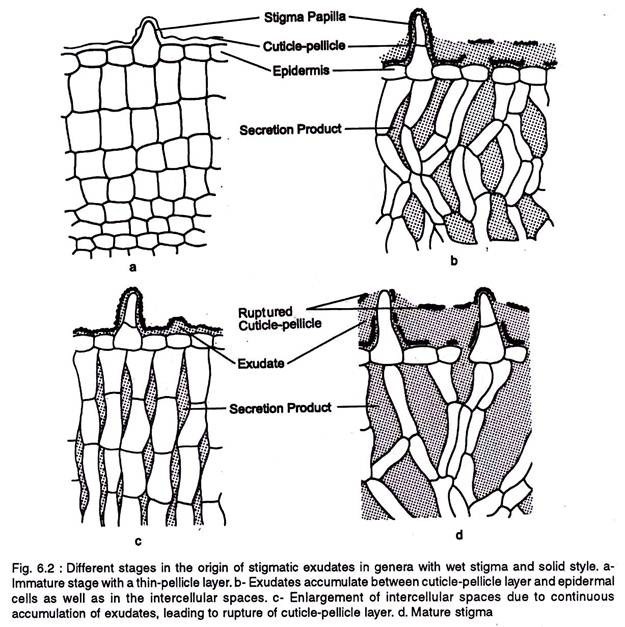
In a wet stigma the exudates originate from the epidermal and subjacent cell layers. The exudates of the subepidermal cells accumulate in the intercellular spaces and form an interconnecting system, while the exudates from the epidermal cells accumulate below the cuticle-pellicle layer (Fig. 6.2).
By continuous secretion of the exudates the intercellular spaces enlarge, as a result the epidermal cells loosen up and the exudates from the subjacent intercellular spaces reach the subcuticular zone. Finally the cuticle-pellicle layer is disrupted releasing the exudates.
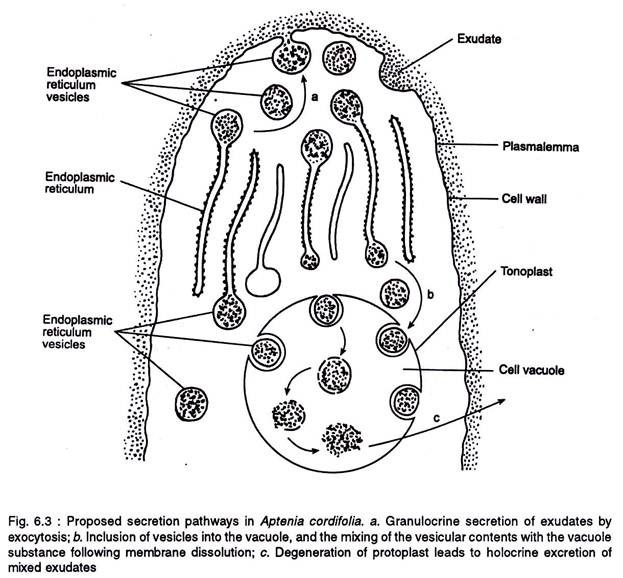
The exudates are secreted by the ER and extruded by exocytosis and the vacuole (Figure 6.3). In the later exudation process some of the ER vesicles are incorporated phagocytotically into the large cell vacuole. The dissolution of the vesicle membrane leads to the mixing up of vesicular material with the vacuolar contents.
Finally the degeneration of the protoplast leads to the holocrine excretion of mixed exudates. The amount of the exudates that accumulate is highly variable and it may be confined to the interstices of papillae or may flood the entire surface. The extracellular components are present in the exudates.
In dry stigma the cuticle is discontinuous and the components of the papillae are extricated through these discontinuities (Fig 6.4 a.) In Crocus the stigmatic secretion accumulates in the chambered stigmatic papillae (Fig 6.4 b).
The grasses have a more elaborate stigma of many layers. Between the pellicle and the cuticle there is a thin mucilaginous layer, and the pectocellulosic (Fig 6.4c) wall is differentiated into several layers, viz., a pectic polysaccharide layer just beneath the cuticle, an electron transparent layer followed by a layer of intermediate electron density that lies just outside the plasmalemma.
Moreover, there is abundance of plasmalemmasomes or paramural bodies (multivesicular bodies) in the papillae, which are, associated with the secretion of surface components, protein, polysaccharide constituents of the petocellulosic wall and intercellular matrix.
Besides the role of stigma in pollen-pistil interaction, the different components of the stigma secretion have also been involved in other functions, viz.:
(i) Lipoidal component is considered to prevent evaporation and wetting by acting as a liquid cuticle,
(ii) Phenolics and proteinase inhibitors have been suggested to give protection against insects and pathogens, and
(iii) The stigmatic exudates serve as a nutrient source for pollinating insects.
The extracellular components are present in the form of a thin extracellular membrane, called pellicle. The pellicle components originate from the epidermal cells of the stigma and/or stigmatic papillae and are extruded onto the surface through discontinuities in the cuticle.
In some cases the pellicle shows ATPase and carbonic anhydrase activity. The pellicle also binds to lectins and contains arabinogalactans, a group of carbohydrates with adhesive properties.
b. The Style:
The style is a tubular structure that connects the stigma with the ovary and is mainly of two types, viz., solid (closed) and hollow (open). The solid style has a central stand of transmitting tissue of elongated cells interconnected by plasmodesmata (Figure 6.5a).
Intercellular substances of pectinaceous nature surround the cells of the transmitting tissue. It also contains proteins, glycoproteins, and often lipids. A number of transmitting tissue-specific proline-rich proteins have been localized in the intercellular matrix.
The cells of the transmitting tissue exhibit normal ultra- structural profiles with numerous mitochondria, active dictyosomes, rough endoplasmic reticulum, plastids and ribosomes. Pollen tubes grow down the style through the intercellular matrix of the transmitting tissue.
In the hollow style the central core has one or more canals which normally correspond to the number of carpels. The stylar canal is bordered by one or a few layers of glandular cells, called canal cells (Fig 6.5 b). A layer of cuticle lines the canal cells in the young bud and the secretion product from the canal cells accumulate below the cuticle.
The canal cells are generally glandular and may be filled with secretion fluid or remain dry. As in solid style system, the stylar secretion in hollow style is rich in carbohydrates and proteins, and shows esterase and acid phosphatase activity.