The following points highlight the five main structures of nucleus and its functions. The structures are: 1. Nuclear Envelope or Karyotheca 2. Nuclear Lamina 3. Nuclear Matrix 4. Nucleoplasm 5. Nucleolus.
Structure # 1.
Nuclear Envelope or Karyotheca:
The nuclear membrane or karyotheca form an envelope-like structure around the nuclear contents and is commonly known as nuclear envelope. The nuclear membrane in higher plant and animal disappears in late prophase during mitosis and re-forms around the daughter chromosomes during telophase. In lower eukaryotes, the nuclear envelope remains intact throughout mitosis.
It separates nucleus from cytoplasm and functions to facilitate and regulate nucleocytoplasmic interaction. The light microscope provides little information about the nuclear envelope. Under electron microscope the nuclear envelope in the interphase or prophase stage appears to consist of two concentric membranes, viz., inner nuclear membrane and outer nuclear membrane.
Each membrane is about 75 to 90 A thick and lipoproteinous in nature. The outer and inner membranes are separated by perinuclear space of 100-170 A0. The inter-membrane space is known as perinuclear cisternae (Fig. 9.4). The inner membrane defines the content of nucleus itself and it contains specific proteins that act as binding sites for the nuclear lamina.
The outer membrane is rough due to presence of ribosomes (25 nm in diameter) attached with it. The ribosomes are engaged in protein synthesis. The proteins made on these ribosomes are transported into the space between the inner and outer nuclear membrane.
In many cells, the outer nuclear membrane is continuous with rough endoplasmic reticulum. The space between the inner and outer nuclear membrane is continuous with the lumen or inner cavity of the rough endoplasmic reticulum.
(i) Nuclear Pore:
The nuclear envelope in all eukaryotes—from yeast to humans—is perforated by nuclear pores. At the margin of each pore, the outer and inner membranes are fused. These pores provide a direct channel between the nucleus and cytoplasm and are universally believed to be the passage way for RNA exit.
In plant cells these are irregularly and rather sparsely distributed over the surface of the nucleus, but in the amphibian oocyte, for example, the pores are numerous and regularly arranged.
The number of nuclear pore ranges from several thousand in somatic cells to several million in large cells such as amphibian oocytes. The nuclear envelope of a typical mammalian cell contains 3,000 to 4,000 proes, i.e., about 11 pores/µm2 of membrane area.
The number of nuclear pores are correlated with the transcriptional activity of the cell. In the frog Xenopus laevis Oocytes (which are very active in transcription) have 60 pores/µm2. But mature erythrocytes (inactive in transcription) of the frog have only 3 pores/ µm2.
The diameter of nuclear pore is about 100 nm. The pores are enclosed by circular and cylindrical structures called annuli. The annulus is an electron dense material. The pores and annuli are collectively called the nuclear pore complex.
Electron micrograph of a negative stained preparation have shown that the nuclear pore complexes have an eight-fold symmetry. Computerised image-processing techniques have shown that the pore complex consists of two rings or annuli with an inside diameter of 80 nm.
Each complex is formed from a set of large protein granules arranged in octagonal pattern (Fig. 9.5). The hole in the centre of each complex often appears to be plugged by a large central granule (Fig. 9.5) attached to the cytoplasmic side.
The central granule may be an inactive ribosome or a newly made ribosome attached to the periphery of the pore complex or other particles caught in transit. Eight radial spokes also extend from plug to rings.
(ii) Nuclear Envelope Protein:
Two proteins have been associated to the nuclear envelope. One is the integral, membrane protein, a glycoprotein of 120,000 Dalton which binds the annuli to the lipid bilayer. The second protein is a 63,000 Dalton protein which binds on the cytoplasmic side of the nuclear envelope. These proteins may be involved in the transport of materials through the nuclear pore.
(iii) Functions of Nuclear Envelope:
1. The nuclear envelope acts as a shield and protects the inner contents of the nuclear compartment.
2. One of the main functions of the nuclear envelope is to prevent the entrance of active ribosomes and other cytoplasmic components.
The nuclear membrane is very selective for the exchange of materials between nucleus and cytoplasm through nuclear pore complexes. The nuclear envelope is very conservative and it does not allow to enter any large cytoplasmic protein or components.
It has been demonstrated by injecting labelled molecules into the cytoplasm and measuring their rate of diffusion into the nucleus. But the nucleus imports many large proteins that it needs such as DNA and RNA polymerase, histone which are synthesised in the cytoplasm.
Again, the nucleus does not export anything to cytoplasm except mRNA and ribosomal sub-units assembled in the nucleolus. The nuclear pore channel is very narrow (about 9 µm in diameter) in resting state. Small molecules can only diffuse in and out through the pore.
How does the large molecules pass through the same nuclear pore? There must be a nucleocytoplasmic traffic system which first sorts out th3 nuclear and cytoplasmic components and gives import or export signal when the pore channel has been induced to enlarge in diameter.
Xenopus oocytes are particularly useful for studying nucleocytoplasmic exchanges because the cells are very large and substances can easily be microinjected into the cytoplasm. When proteins are extracted from the nucleus of Xenopus oocyte and microinjected back into the cytoplasm; all protein molecules, including larger one (having a diameter of up to 20 nm) easily re-accumulate in the nucleus from the cytoplasm.
It has been shown that the nucleus contains huge amount of a specific protein called nucleoplasmin which carries the nuclear signal for nucleocytoplasmic exchange. Nucleoplasmin is a large pentameric nuclear protein with a distinct head and tail domains.
The heads can be cleaved from tail by limited proteolysis. When intact nucleoplasmin is microinjected into the cytoplasm of a frog oocyte, it rapidly re-accumulates in the nucleus even though they are too large to diffuse passively through the small channel of a nuclear pore complex.
When proteolytically cleaved nucleoplasmin is microinjected into the cytoplasm, only tail pieces are taken up by nuclei but head pieces are not (Fig. 9.6). This is detected by autoradiography using radioactive intact nucleoplasmin as well as cleaved radioactive head and tail pieces of nucleoplasmin.
Again, when isolated tails of nucleoplasmin are linked with 20 nm diameter colloidal gold spheres—which are much larger than the inner diameter of the resting nuclear pores— and are microinjected into the cytoplasm of frog oocyte, the gold spheres accumulate in the nucleus and are seen in the nuclear pore during transport by electron micrograph.
This experiment demonstrates that the tail piece of nucleoplasmin is karyophilic protein and contains a signal mechanism which, when attached to bigger molecules (even metal particle like gold), helps to transport into the nucleus from cytoplasm through nuclear pores.
In response to such karyophilic signal protein, the nuclear pores can open up to (i.e., tail of nucleoplasmin) accommodate an object as large as a gold particle. Otherwise, in absence of signal mechanism, the nuclear pore remains open up to its resting stage.
It also indicates that the nuclear pore appears to function like a close- fitting diaphragm that opens up to right extent when activated by signal on an appropriate large protein. How this occurs at the molecular level was unknown for many years.
Recently, it has been possible to identify the specific karyophilic signal segment on nuclear protein tail using gene manipulation techniques by progressively deleting parts of gene. On the other hand, the nuclear pore contains one or more receptors that recognise nuclear protein import signal and provides a direct passage for inward transport of nuclear protein bound macromolecules from cytoplasm to nucleus.
It is found that the SV40 virus, when it enters an eukaryotic cell, encodes a large protein molecule called T-antigen that is needed for viral DNA replication in host nucleus. The T-antigen normally migrates without any association of nuclear protein in the host nucleus shortly after being synthesised in the host cytoplasm.
Therefore, it indicates that the T-antigen definitely contains a signal segment similar to tail-piece of nucleoplasmin and such segment helps to migrate the giant T-antigen molecule into the host nucleus via nuclear pores. However, a mutation in a single amino acid of T-antigen prevents its nuclear transport via nuclear pore and causes the mutant protein to remain idle in the cytoplasm.
From the above observation it is determined that this mutation occurs in the short lengths of DNA encoding region of normal T-antigen. This observation again indicates that the mutated region of DNA definitely encodes the small segment of T-antigen which acts as signal sequence for the migration of T-antigen into the cell nucleus.
Later it was found that the karyophilic signal sequence of T-antigen is very simple, positively charged and short and it is made of Thr-Lys-Lys-Arg-Lys-Pro which are located in an internal region of the T-antigen polypeptide.
Thus, it seems that the tail of nucleoplasmin also contains such similar signal sequence of amino acid which are recognised by the pore receptors. So the above experimental observations explain how the macromolecules migrate from cytoplasm to nucleus.
On the other hand, ribosome sub-units, mRNA, are produced in the nucleus but function in the cytoplasm. Are they transported out of the nucleus by the same process or is there any other mechanism or nuclear signal? It is another set of questions.
Using the same experimental technique on frog oocyte, it is observed that when isolated small RNA molecules (tRNA or 5S RNA) coated with nucleoplasmin are injected into the nucleus, they are rapidly transported through pore into the cytoplasm. But, if they are injected into the cytoplasm, they remain there.
It seems that nuclear pore contains another receptor(s) which recognises nuclear protein export signal. The export signal sequence may be present either on the same nucleoplasmin polypeptide at different locus, or on the other special nuclear protein.
It is also known that the mRNA makes a complex with special nuclear protein to form ribonucleoprotein particles and are thought to be actively exported from the nucleus. When ribonucleoprotein comes out, mRNA sets free from protein part.
The receptor present at the pore catalyses active transport outward instead inward. Probably ribosomal sub-units are actively transported outward through the pores by the similar fashion.
Structure # 2.
Nuclear Lamina:
The nuclear lamina is a protein meshwork that lines the inner surface of the inner nuclear membrane in interphase cell forming a discrete layer of 30-100 nm thick that connects the inner membrane with chromatin. It is composed of three principal extrinsic membrane proteins, lamins A, B and C—which together forms a fibrous network.
The lamins are made up of two parts-—one is rod-like tail of 52 nm long and other is two globular heads at one end. Lamins are highly similar in sequence and structure with the cytoplasmic intermediate filament (IF). Lamins A and C are identical except for the presence of an additional 133 amino acids at the carboxyl end of Lamin A. Lamin B appears to have a specialised membrane binding role.
Inner nuclear membrane contains a lamin B receptors that binds specifically to lamin B. Lamins A and C bind with lamin B and mediate interactions between the lamina and chromatin.
(i) Function of Lamina:
The lamina with its lamin polypeptide carry out the following functions:
1. Regulating assembly and disassembly of the nuclear membrane during cell division.
2. Attachment of chromatin to the nuclear envelope.
3. Helps to form micronuclei when the cells are left for a long time in colchicine.
(ii) Assembly and Disassembly of Nuclear Membrane:
From the light microscopic observation, it is known that the nuclear envelope disappears in late prophase during cell division when the chromatin condenses into chromosome and reappears around the daughter chromosomes during telophase. Disappearance and reappearance of nuclear membrane during cell division are also correlated with disassembly and assembly of nuclear lamina.
Immunofluorescence studies have shown that at the onset of prophase the lamins start to disassembly and appear in the cytoplasm. Disassembly of the lamina takes place due to de-polymerisation. Biochemical studies have shown that the de-polymerisation of lamina is due to phosphorylation.
The phosphorylation reaction is catalysed by an enzyme, lamin kinase. This whole process, in turn, causes the nuclear envelope to disassembly into a number of small vesicles that disperse into the cytoplasm. The electron microscope, however, shows that lamin B remains attach with these vesicles, whereas lamins A and C are de-polymerised to small oligomer and dispersed into the cytoplasm.
During telophase, after the daughter chromosomes have separated and begin to de-condense, nuclear assembly is started. This process induces the de-phosphorylation and polymerisation of lamins into a fibrous network and simultaneously small nuclear vesicles fuse with each other (Fig. 9.7).
Polymerised lamin B associated with fused vesicle in turn binds with polymerised lamins A and C and the whole process makes a bridge between the chromatin and fused small vesicle of nuclear envelope.
In frog embryos and some other cells, the nuclear structure reassembles in two-step process—nuclear membrane first form around individual chromosomes and then fuse together to form a single interphase nucleus. How and when pore complex form is not known.
(iii) Attachment of Chromatin with Nuclear Envelope:
One of the lamina proteins, particularly, lamin B, has got the strong affinity to bind with nuclear envelope on one hand and with polymerised lamins A and C on the other hand. Actually, lamin B acts as a linker between nuclear envelope and other lamina proteins.
However, it does not have any binding ability with chromatin. Whereas, lamins A and C binds strongly with chromatin because they are the most abundant DNA-binding proteins in most eukaryotic nuclei. Thus, nuclear lamina may play a crucial role for the attachment of chromatin with nuclear envelope.
(iv) Formation of Micronuclei:
In fact, when the cells are kept for a long time in colchicine to arrest mitotic metaphase, the lamins assemble around individual chromosome which, in turn, become surrounded by nuclear envelopes giving rise to micronuclei containing one chromosome.
Structure # 3.
Nuclear Matrix:
Nuclear matrix or scaffold is an intra-nuclear framework, analogous to the cytoskeleton. In electron micrographs of nuclei from which all DNA has been removed, a network of protein fibre is left inside the nucleus. This structure is called the nuclear matrix or scaffold.
It may also be defined as the insoluble material left in the nucleus after a series of biochemical extraction steps. The proteins that constitute the matrix can be shown to bind specific DNA sequences called SARs or MARs (for scaffold or matrix-associated regions).
A number of experiments also suggest that hn-RNA and partially processed transcripts are also present in the matrix. The structural components of matrix have not yet been fully identified.
Function of Nuclear Matrix:
The nuclear matrix performs the following functions:
1. It is responsible for the determination of nuclear shape.
2. It gives the mechanical support to resist disaggregation of nucleus in high ionic strength buffers.
3. The matrix may help to organize chromosomes, to localize genes and to regulate DNA transcription and replication.
4. One important possibility is that the protein fibres form a pathway out of the internal regions of the nucleus to allow mRNA to reach the nuclear surface.
Structure # 4.
Nucleoplasm:
The transparent, semisolid, granular and slightly acidophilic ground substance of the nucleus is known as nucleoplasm. The nuclear components such as the chromatin threads, nuclear matrix and the nucleolus remain suspended in the nucleoplasm.
Nucleoplasm is composed mainly of nucleoproteins but it also contains various inorganic and organic substances. DNA and RNA are the common nucleic acids of nucleoplasm. The nucleoplasm contains many enzymes which are necessary for the synthesis of DNA and RNA. Nucleoplasm carries out the biosynthetic functions of the nucleus.
(i) Significance of Nucleus:
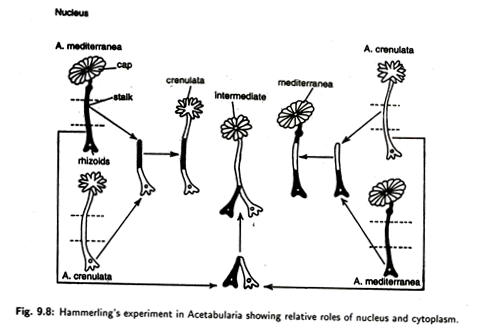
Nucleus has its main role in determining the growth and development of the morphological character and biochemical events of a cell. In 1934, J. Hammerling, a German biologist, demonstrated that the nucleus determines the characters of the cell and, ultimately, the characters of the individual. He provided the evidence for this while doing experiments on the genus Acetabularia sp.—an unicellular green algae (Fig. 9.8).
The species of this genus show a body which is divided into three parts like a long cylindrical stalk, a rhizoidal portion at the bottom of the stalk and an umbrella-like cap at the top of the stalk. The nucleus of this species is situated in the rhizoid. If the cap is cut off, the nucleated rhizoid with stalk again develops a cap of its own kind.
In another experiment Hammerling took one species (A. crenulata) with lobed margin of the cap and another species (A. mediterranea) having cap with entire margins. If, after removing the caps, the stalk of one species is grafted on the rhizoid of the other species, and vice versa, thus making the mutual exchange of nuclei between two species, the shape of the cap will be determined by the nucleus and not by the stalk.
It means that if the nucleus belongs to A. crenulata, shape of the cap will be of the cranulata type. If the nucleus comes from A. mediterranea, cap will be of mediterranea type. However, when the nucleated rhizoidal fragments of the both species are hybridized, then the shape of the cap will be intermediate type.
The conclusion drawn from such experiments is that the nucleus produces material that comes out in the cytoplasm of the stalk or rhizoid and participates in the control of cell growth and, ultimately, influences the morphology of the regenerated cap.
Therefore, the crucial finding is that the characteristics of an individual are controlled by the nucleus of the cell. But the experiments with hybrid fragments containing the nuclei of both species show that the interaction of different kinds of nuclei have also a determining role of the type of cap to be developed.
Structure # 5.
Nucleolus:
The nucleolus is the only organised body present in the nucleus (Fig. 9.9). In course of cell division, it usually disappears by late prophase, reforms during telophase and retains up to late prophase of the next cell cycle (Fig. 9.10).
In certain cases, it may persist till metaphase or later (e.g., Spirogyra, Pea and a number of animal cells in culture). Under the light microscope the suitably stained nucleolus is seen as a dense body of variable size and shape.
Its presence in the nucleus was first recognised by Fontana in 1874. The nucleolus is formed in the nucleolar organising region of the chromosome(s). It is now well-established that the nucleolus is the site for the synthesis and biogenesis of ribosomal nucleic acid (rRNA) in eukaryotes.
Nucleolus is the small intra-nuclear dense globular organised body disappearing by late prophase and reforming at tele-phase in the nucleolar organising region of the chromosome(s) and is the side for the synthesis and biogenesis of ribosomal ribonucleic acid.
(i) Brief Past History of Nucleolus:
In the historical background of the nucleolus, Ogata (1883) recognised two types of nucleoli within nucleus. One is called acidophilic plasmosome or real nucleoli and the other is basophilic nucleoli or karyosomes consisting of chromatin. In 1898 Montgomery described the structure of nucleolus as a homogeneous spheroid intra-nuclear body.
McGill (1906) described that there were two regions of a nucleolus. The central region is oxyphilic area which is surrounded by basophilic area. Belar (1928) first noted that the nucleoli were often associated with chromosome. Nucleolus-chromosome association was first established by Heitz (1931).
Casperson etal (1940, 1942) demonstrated the presence of RNA in the nucleolus. By methyl green pyronin staining, Brachet (1942) showed that the nucleolus was rich in RNA. In 1950 Godward noted the presence of filamentous structure within the nucleolus.
The filamentous structure shows a positive Feulgen reaction and is considered a functionally active part of special chromosome (Lettr’e and Siebs, 1954). Estable and Sofelo (1951), using a silver impregnation technique, reported the presence of the filamentous component as ‘nucleonema’ and a structureless ‘pars amorpha’ within the nucleolus.
(ii) Structure of Nucleolus:
Under light microscope, the nucleolus is seen as a spheroidal sub-organelle in the nucleus that is not dividing (Fig. 9.11). The relative size and shape and the number of nucleolus can only be observed with no further details. Some of the details of nucleolus can be seen in the electron microscope. Unlike the cytoplasmic organelles the nucleolus is not bounded by a membrane.
In a typical electron micrograph, three regions within the nucleolus can be distinguished:
(i) Ribonucleoprotein (RNP) fibrils,
(ii) RNP granules and
(iii) Chromatin elements.
In active nucleoli all three components are intermingled with each other.
(a) Ribonucleoprotein (RNP) Fibrils:
It is the fibrillar component of the nucleolus. The average diameter and length of RNP fibrils are 4-8 nm and 20-40 nm, respectively. The RNP fibrils can be digested with the treatment of RNase and show a strong affinity with silver indicating that they are made of ribonucleoprotein.
(b) Ribonucleoprotein Granules:
It is the granular components with 15-20 nm in diameter. At high magnifications, the granules look like a vesicle with a light central core and a dense peripheral boundary. They are apparently similar to cytoplasmic ribosomes.
Some workers consider that the granular component contains maturing ribosomal precursor particles. These components can be digested partially with RNase and the remaining parts disappear by the treatment of pepsin. Therefore, it indicates that they are made of ribonucleic acid and protein.
(c) Chromatin Elements:
The presence of chromatin elements in the nucleolus remained controversial for long time. It is found that the periphery of nucleolus is surrounded by the prominent chromatin element like a shell. It is designated by some authors as a perinucleolar chromatin (Fig. 9.11).
These chromatin elements are highly condensed and do not take part in the formation of the nucleolus. It is devoid of ribosomal gene or cistron (r-DNA). According to Lima-de-Faria etal (1969), a very small percentage of DNA of the nucleolus associated chromatin elements of certain insect oocytes contains r-DNA.
In the recent year it has been claimed that apart from the nucleolus associated chromatin elements, some other chromatin fibrils are also present within the nucleolus. It is called as the intranudeolar chromatin or the fibrillar centre. The fibrillar centre has got a dense peripheral fibrillar component.
The fibrillar centre also contains proteins, i.e., phosphoprotein C23. The presence of r-DNA and localisation of RNA polymerase activity at the fibrillar region have also been demonstrated by different techniques.
Knibiehler etal (1977) and Mirre and Stahl (1981) reported that the transcriptional activity of the r-DNA has been found at the periphery of fibrillar centre on dense fibrillar component but not on the fibrillar centre themselves. It seems that the ribosomal cistron (r-DNA) may be present in both these regions.
However, the fibrillar centres are not present in all cell types. The nucleolus of rat hepatocyte is devoid of fibrillar centre and the activity of RNA polymerase I is detected in the fibrillar component of the nucleolus.
The nucleolus-associated chromatin elements is generally absent in plant nucleoli. But the chromatin in extended form possibly belonging to the NOR can be found penetrating the nucleolus. This chromatin is surrounded by a zone of ribonucleoprotein fibril.
On the outside, the fibrils are again found with granules followed by a zone consisting exclusively intermingled of RNP granules. In highly active cell this arrangement is lost. Plant nucleoli sometimes contains more or less spherical light areas of lower density than the surrounding nucleolar mass. These are called as nuclear vacuoles.
A nuclear skeleton remains associated with the nuclear matrix network which is made of acidic proteins. This protein is specifically associated with the nucleolus and is chemically and immunologically different from the other nuclear matrix. Therefore, the nucleoli seem to have an independent skeletal structure.
(iii) Number, Size and Shape:
The nucleolus is absent in bacteria, yeast, in some algae, undifferentiated embryonic cells of amphibians and in certain mammalian cells like immature erythrocytes, reticulocytes and spermatozoa. In a nucleus, one or many nucleoli may be present.
In mature stage of lymphocytes plasmocytes and monocytes, the nucleolus is smaller in size and have a ring-shaped appearance which is probably due to the inhibition of RNA synthesis.
In the nerve cell, number and size of nucleolus depends on the age and functional stage. Ultra-structurally, nerve cell nucleoli often show trabecular structures surrounded by small light areas containing granules. Cytochemical and morphological studies suggest that the nucleolus in nerve cell is possibly responsible for the synthesis of cytoplasmic Nissl substance.
Sometimes nerve cells in female animals bear a Feulgen-positive nucleolar satellite which is called sex chromatin body. This sex chromatin body may not always be associated with the nucleolus.
In malignant tumour cells, nucleoli vary widely in respect to size, shape and number. The nucleoli are enlarged and compact with granular elements and are often irregular in shape. In general, nucleolar size and number are possibly related to the numerical changes of chromosome with tumour progression.
In plants, changes in number, size and shape of nucleoli have been reported in differentiating cells. In non-dividing cell the nucleoli are small and compact whereas, in dividing cell, it is large and vesicular in shape. Large number of small fibrillar centres and abundant granular components of highly active cells are progressively lost with a slowing down of the metabolic activity.
The size of nucleolus changes in relation to starch grain formation in maize. With the progress of differentiation the small nucleoli of the tuber cells of Helianthus grow into large size. But the reverse process is seen during the differentiation of xylem vessels in corn leaves.
(iv) Formation of Nucleolus:
The development of nucleolus in the restituting nuclei is known as nucleogenesis. The nucleolar organizer or a specific nucleolar organising region of a chromosome (NOR) is responsible for the production of nucleolus. Each NOR can form a single nucleolus or several NORs can cooperate to form one nucleolus.
When the NOR in a chromosome is intercalary in position, it is generally known as secondary construction. This region is narrow and less dense. The terminal NOR, however, does not produce any secondary constriction. The terminal NORs particularly in man often form nucleoli. The number of nucleolar chromosome is specific for a species.
Experiments with amphibian cells have shown that there is a genetic basis for the nucleolar formation. The genetic control of nucleolus formation has also been demonstrated in hybrids of the plant Crepis. Each species of Crepis has its own specific nucleolar organizer. When two species are hybridized, the nucleolar organizers show the differential activity in the hybrid cell.
One remains functional and the other remains inactive by differential repression of gene. When the inactive one is shifted to the original condition, it again becomes active. The cistrons coding for ribosomal RNA remained grouped in particular segments of chromosome which are also involved in the organisation of nuclei.
Nucleolus first appears in the cell as droplets, which increases in size as the cells synthesize more RNA and accumulate more proteins. All the tiny nucleolus like bodies which can be observed in telophase, are not necessarily new synthetic products. Many of them are nucleolar remnants from the preceding interphase. They remain entrapped in the chromosome mass and finally released at telophase as droplets.
The nucleolar remnants are often attached to various segments of chromosome, all of which may not be associated with the formation of the nucleolus. Nucleolar RNA synthesised prior to mitosis and simultaneous nucleolar RNA synthesis in post mitotic nuclei are incorporated in the newly formed nucleolus. As the nucleolus grows in size, the chromosome threads are pushed apart.
The chromatin inside the nucleolus uncoils and becomes thinner and finally disintegrates within the nucleolus. The nucleolus rapidly enlarges in size in early interphase due to both internal growth but the further increase of nucleolus in size may take place in late interphase due to fusion of smaller nucleoli.
(a) DNA Configuration of Nucleolar Organizer:
The DNA axis isolated from nucleolus or nucleolar organizer contains multiple copies of rRNA genes that code for ribosomal RNA. Human cells contain about 200 rRNA gene copies per haploid genome. In case of Xenopus cell contains about 600 rRNA gene copies per haploid genome. These genes are tandemly repeated along the DNA molecule (in a head to tail arrangement).
The ribosomal transcription unit (RTU) contains the genes for 18S, 5.8S and 28S rRNA (Fig. 9.12) separated from each other by internal transcribed spacers (ITS) and flanked on its 5′ side by an external transcribed spacer (ETS). The transcript units themselves are also clustered in tandem repeats, each unit being separated from the next by a non- transcribed spacer (NTS). The spacers can vary greatly length and sequence.
The internal transcribed spacer is copied into RNA but does not give rise to mature rRNA. The rRNA genes axe transcribed by RNA polymerase I. The RNA polymerase molecules and their associated transcripts (rRNA) are so densely packed that the transcripts fan out perpendicularly from the DNA to give each transcription unit a Christmas tree appearance (Fig. 9.13).
The tip of each of these trees is the point on the DNA at which transcription starts and, where the transcripts axe shortest, while the other end is the termination site of transcription and transcripts are longest (Fig. 9.13).
The external transcribed spaces behaves like promoter. This promoter-like sequence contains between -72 and -114 a region necessary for transcription which provides a binding site for protein factor. The protein factor is species specific and it is also known as transcription factor.
On the right side of protein factor binding site, there is a RNA polymerase I binding site from, where RNA polymerase I moves to transcribe the rRNA genes (Fig. 9.14).
The protein factor is needed to increase the binding ability of RNA polymerase I at RNA polymerase I binding site. That means, when protein factor binds at factor binding site, it acts as enhancer for the binding of RNA polymerase I at RNA polymerase I binding site. Therefore, the external transcribed spacer ac as “sinks” for RNA polymerase I.
The main component of NTS contains several repeats of 60-80 bp which has a striking similarity or homology with promotor like sequence (PLS). In addition, NTS contains some duplication of the entire PLS (Fig. 9.15).
As a result, RNA polymerase I is attracted by the various points of NTS. Sometimes transcription can start from the duplicated promotor. Just to save the false entry of RNA polymerase I at the imperfect position of NTS and to stop any transcripts that might start in the NTS, there is a row of poly-T nucleotides situated before 225 nucleotide before the PLS. That Poly T sequence acts as a fail safe terminator and stop them.
(v) Functions of the Nucleolus:
Although various functions have been assigned to nucleolus, presently it is believed that the nucleolus is the most important site for ribosomal RNA (rRNA) synthesis and biogenesis of ribosomes.
This has been proved by some important findings:
1. Rapid protein synthesis is correlated with a corresponding increase in nucleolar volume.
2. In the electron microscope, the particles present within the nucleolus are very similar to ribosomes. These particles have the identical sedimentation characteristic of ribosome.
3. The nucleotide composition of nucleolar RNA is closely identical with that of cytoplasmic RNA.
4. Inhibition of nucleolar RNA synthesis by low concentration of Actinomycin D or inactivation of the nucleolus by UV rays also prevents further accumulation of cytoplasmic ribosomal RNA.
5. Anucleolate mutant form of Xenopus laevis (an African clawed toad) does not have any nucleolus in the embryo genic cell, where ribosomal RNA synthesis is almost nil. So, embryonic lethality is found in that mutant form. Therefore, it once again proves that the nucleolus is the site for rRNA synthesis.
6. 28S rRNA and 18S rRNA can be selectively hybridized with nucleolar DNA. It indicates that rRNA is complementary to the nucleolar DNA.
(a) Synthesis of rRNA:
It is evident that r-DNA present within nucleolus or nucleolar organising region contains many copies of rRNA genes which are the site for the synthesis of ribosomal precursor RNAs. The transcription of rRNA genes are catalysed by RNA polymerase I enzyme.
RNA polymerase-I is located in the nucleolus and is responsible for the synthesis of ribosomal precursor RNAs which are not translated into protein. But they first undergo some modifications and involve in the biogenesis of ribosome in association with proteins. How RNA polymerase-I begins and ends transcription of rRNA genes were not known for many years.
It has been demonstrated that, when purified, r-DNA is incubated with RNA polymerase-I and necessary nucleotides, no ribosomal precursor RNA is synthesised. However, if a small amount of crude nuclear extract is added, transcription of rRNA genes begins.
The analysis of purified nuclear extract shows that it contains two r-DNA-binding factors that assist RNA polymerase-I to do work correctly and help transcription of rRNA genes. It is known that RNA polymerases do not recognize the promotor on purified DNA molecules.
Instead, it needs the help of one or more sequence- specific DNA binding factors to become functional on DNA. In case of RNA polymerase I one of the factors is B factor and other is S factor. B factors can bind to r-DNA by itself and its binding affinity is increased in the presence of S-factor.
The two factors bind first, then RNA polymerase I; transcription begins once this initiation complex is formed. The B and S factors are species-specific and are protein in nature.
The initiation site for transcription lies upstream of the 5′ end. Once the RNA polymerase-I starts the transcription, it does not stop, can move efficiently from one transcription unit to the next one so that transcription is not interrupted. The RNA polymerase-I does not encode NTS (Fig. 9.16).
(b) Biogenesis of Ribosome:
Ribosomes are spheroidal particles that contain approximately equal amounts of rRNA and protein. They are found in all cells and are involved in protein synthesis. Biogenesis of ribosomes takes place in the nucleolus and involves several distinct steps that lead to formation of the 40S and 60S ribosomal sub-units in higher organisms.
The rRNA genes or ribosomal transcription units are at first transcribed by RNA polymerase I and each transcription unit produces the same primary transcripts, known as 45S rRNA (13,000 nucleotide long) that contains the 18S (about 2,000 nucleotides), 5.8S (about 160 nucleotides) and 28S rRNAs (about 5,000 nucleotides) joined to each other by transcripts of the transcribed spacer.
This large rRNA molecules is known as ribosomal precursor RNA (Fig. 9.17).
The ribosomal precursor 45S RNA then combine with proteins to form 80S ribonucleoprotein particles (RNP). It may contain ribosomal and other accessory types of proteins that are made in the cytoplasm and are transported to nucleolus via nucleus.
Methylation of 18S and 28S rRNA regions of the 45S molecule also occurs during its transcription. In this process, methionine acts as methyl donor. Methylation is required for capping the cut pieces of precursor molecule and it stabilizes the 18S, 5.8S and 28S rRNA during cleavage.
In step I, some non-methylated and short-lived intermediates of the spacers are removed from 80S ribonucleoprotein complex containing 45S rRNA.
In step II, 80S RNP complex is subsequently split into one small sub-unit containing 18S rRNA and accessory protein and one large sub-unit containing 32S rRNA plus accessory protein.
In step III, the large sub-units containing 32S rRNA also cleave into 28S and 5.8S rRNA and they remain hydrogen bonded.
In step IV, the small sub-units containing 18S rRNA plus accessory protein form the 40S particles are rapidly exported to the cytoplasm than the large (60S) ribosomal sub-units.
In step V, an immature large sub-unit containing 28S and 5.8S rRNA plus accessory protein ultimately forms the large ribosomal sub-units when another type of small 5S rRNA is incorporated. The large ribosomal sub-unit is made of 28S, 5.8S and 5S rRNA and accessory proteins. The transcription unit of this 5S rRNA are not located in the nucleolus.
Therefore, the synthesis of 5S rRNA occurs outside of the nucleolus. For instance, the approximately 160 repeats of the Drosophila 5S gene appear to be arranged in a single cluster on chromosome 2, while those of Xenopus occur at the telomeres of most—if not all—chromosomes. The 5S rRNA genes are transcribed by RNA polymerase III.
A transcription factor called TF III A binds to the gene and enables RNA polymerase III to start transcription at an upstream site.
Once the large sub-units of ribosome matures, they are released from the nucleolus and exit into cytoplasm through the nuclear pores (Fig. 9.18). In cytoplasm the two sub- units remain free and inactive. During protein synthesis, they join with each other and become active.
Active ribosomes are also often found in clusters and under these conditions are known as polyribosomes or polysomes. The polyribosomes are held together by a string of mRNA during its translation.
Fig. 9.18 : The function of the nucleolus in ribosome synthesis
Synthesis of rRNA and Biogenesis of Ribosomes in Bacteria (Prokaryotes):
True nucleus and nucleolus are absent in bacteria. But its cell contains ribosomes. Naturally the question arises: How ribosomes of the prokaryotic cells are synthesised without nucleolus? Bacterial cell contains 70S type ribosome which is made of two sub-units such as 30S and 508.
The small sub-unit or 30S sub-unit consists of 16S rRNA and ribosomal proteins whereas large sub-unit or 50S sub-unit contains 23S rRNA and 5S rRNA plus ribosomal proteins.
The genes for three species of rRNA and one tRNA are clustered at a segment of a single bacterial chromosome or DNA and are transcribed as a single unit. Endonucleolytic cleavage of this transcript releases individual molecules of rRNA and tRNA.
However, in some eubacteria, the arrangement of such genes is slightly different and, in the case of rRNA genes, the 18S and 28S genes axe transcribed as a unit (along with one tRNA gene) that does not include the gene for the 5S rRNA which is transcribed separately.
Most of the Archaebacteria like the eubacteria have the three rRNA genes clustered and transcribed together; however, these transcription units do not contain a tRNA gene. A cluster of genes for ribosomal protein also occupy space on the same bacterial chromosome adjacent to rRNA genes.
Ribosomal protein molecules bind to the cleaved transcripts of rRNA in the process of ribosomal self-assembly which takes place directly in the cytoplasm and finally form the sub-units of ribosome (Fig. 9.19).
By some still unknown mechanism an excess of ribosomes inhibits transcription of rRNA genes. If the ribosomal proteins are synthesised more rapidly than they are utilised in assembly they inhibit translation of their encoding mRNA molecules.
Thus, under the regulation of these controls, ribosomes are synthesised in response to cellular requirements for their use and synthesis of their components is coordinated. An additional mechanism prevents over-synthesis of rRNA following inhibiting protein synthesis by a sudden deprivation of an amino acid.
Synthesis of rRNA and Biogenesis of Ribosomes in Mitochondria and Chloroplast of the Eukaryotic Cells:
In addition to characteristic 80S cytoplasmic ribosomes, eukaryotes have also ribosomes in their mitochondria and chloroplasts that resemble 70S ribosome of prokaryotes more closely than they do 80S ribosomes. Both mitochondria and chloroplast do not have any nucleolus or nucleolus like bodies.
Therefore, the synthesis of organelle specific rRNA and the biogenesis of ribosome take place more or less like that of prokaryotes.
Fundamentally, mitochondrial DNA (mt-DNA) is circular and made of an outer H(heavy) strand and inner L(light) strand. The number, size and the arrangement of genes of mt-DNA are very different in various organisms.
The ribosome of mitochondria is made of 30S and 50S sub-units. Smaller sub-units or 30S contains 12S rRNA and large sub-units contain 16S rRNA. The 5S rRNAs are not found in the ribosomes of mitochondria. The genes that encode 12S and 16S rRNA as well as a number of tRNA may be either clustered at a particular segment on H-DNA strand or dispersed at different loci of H-strand (Fig. 9.20).
RNA polymerases and most of the ribosomal proteins are encoded by the nuclear DNA and imported from the cytoplasm to mitochondria. Yeast and fungal mt-DNAs encode one ribosomal protein (termed var-I) not present in human mt-DNA.
Transcription of H-strand of human mt-DNA is initiated at two sites. Primary transcript I initiates just upstream of the tRNAPhe gene and terminates just after the 16S rRNA, it is processed by endoribonucleases to yield one molecule of tRNA. The rRNAs finally bind with ribosome proteins and form the mitochondrial ribosomes that remain in the mitochondria.
The chloroplast ribosomes are typically bacterial in terms of the sizes of the individual rRNA molecules (5S, 16S and 23S), total ribosome size (about 70S), sub-unit size (30S and 50S) and total number of ribosomal proteins.
120 genes, about 60 genes are involved in RNA transcription and translation including genes for rRNAs, tRNA, RNA polymerase sub- units and ribosomal proteins. The liverwort (Marchantia polymorpha) chloroplast genome has two inverted repeats such as IRa and IRb that contains the rRNA genes (Fig. 9.21).
These repeats are separated by two single copy sequences, one small and one large, that contain the bulk of the tRNA and protein coding genes. The chloroplast genome also contains genes such as rpl and rps that encode 8 proteins for 50S sub-units, respectively.
The smaller sub-unit or 30S contains 16S rRNA transcript that combines with 11 ribosomal proteins whereas the large sub-unit or 50S contains 23S and 5S RNA transcript plus 8 ribosomal proteins.
(c) Other Functions:
Although it is well-established that r-DNA is responsible for the ribosomal RNA synthesis, only a few cistrons are required for the substance of the normal activity of the nucleolus.
It has also been suggested that the nucleolus may in some way be linked to the nuclear RNA transport.