Anaerobic respiration is an alternate mode of energy generation in which an exogenous electron acceptor other than O2 is used in electron transport chain leading to a proton motive force.
In contrast to aerobic respiration where O2 is used as electron acceptor, the electron acceptors used in anaerobic respiration include nitrate (NO–,3)), sulphate (SO2-4), carbonate (CO2), ferric ion (Fe3+), and even certain organic compounds (e.g., fumarate, chlorate, trimethylamine oxide, etc.)
The use of these alternate electron acceptors allows microorganisms to respire in environments where oxygen is absent. Because the solubility of oxygen in water is rather low, and because oxygen is in such high demand as an electron acceptor by aerobic organisms, anaerobic respirations are thus ecologically extremely important for anaerobic organisms, the prokaryotes. Anaerobic respiration, therefore, is a hallmark of prokaryotes and is rare in eukaryotes.
Obligate Anaerobes and Facultative Aerobes:
Most of the microorganisms are obligate anaerobes because they carry out only anaerobic respiration and are unable to use O2. They are so sensitive to oxygen that they are inhibited or even killed by oxygen. So far as is known, obligate anaerobes belong to three groups of microorganisms: a wide variety of prokaryotes, a few fungi, and a few protozoa.
One of the best-known group of obligately anaerobic bacteria belongs to the genus Clostridium, which are widespread in soil, lake sediments, and intestinal tracts and are often responsible for spoilage of canned foods.
Other obligately anaerobic bacteria occur among the methanogens (e.g., Methanobacterium formicicum) and many other species of archaebacteria, sulphate reducing and homoacetogenic bacteria, and many of the bacteria present in the animal gut.
Among obligate anaerobes, there are some bacteria that have ability to tolerate oxygen and grow in its presence even though they cannot use it. Such obligate anaerobes are called aerotolerant anaerobes (e.g., Streptococcus pyogenes).
Facultative aerobes are those bacteria that carry out aerobic respiration if oxygen is present in the environment, but switch over to an alternate electron acceptor to carry out anaerobic respiration when oxygen is depleted from the environment. An example of facultative aerobe is Escherichia coli.
Assimilative and Dissimilative Metabolism:
All microorganisms need sources of N, S, and C for growth. When an inorganic compound such as nitrate (NO–3), sulphate (SO2-4) or CO2 is reduced for use as a nutrient source, it is said to be ‘assimilated’ and the reduction process is called assimilative metabolism.
But when such inorganic compounds are used as electron acceptors for energy metabolism in anaerobic respiration, they are said to be ‘dissimilated”, and the reduction process is called dissimilative metabolism.
Assimilative and dissimilative metabolisms differ markedly from one another. In assimilative metabolism, only comparatively lesser amount of the compounds (NO–3 SO2-4or CO2) is reduced to fulfill the requirements for cell growth and reduced atoms are eventually converted to macromolecules that constitute the cell material.
In dissimilative metabolism, a comparatively large amount of electron acceptors (NO–3 SO2-4 or CO2-3, etc.) are reduced, and the reduced product is excreted by the organism into the environment as waste product.
Assimilative metabolism is carried out by many organisms (many bacteria, archaebacteria, fungi, algae, and higher plants), whereas only a restricted variety of organisms, primarily the prokaryotic microorganisms, carry out dissimilative metabolism.
Common Types of Anaerobic Respiration:
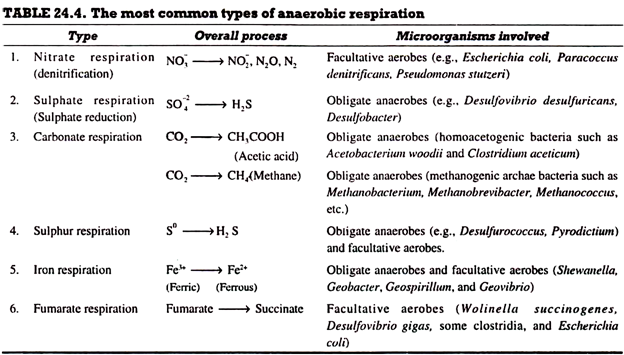
Since a variety of compounds are used as exogenous electron acceptors by anaerobic microorganisms during dissimilative metabolism for energy conservation because of their varying ecological behaviours (obligate and facultative), they have adopted varying routes for anaerobic respiration. In the light of this, the anaerobic respiration can be categorized into different types. A summary of the most common types of anaerobic respiration is given in Table 24.4.
Nitrate (NO–3) Respiration (Nitrate Reduction):
Nitrate (NO–3) is one of the most common type of inorganic electron acceptor used in anaerobic respiration and is reduced to NO–2, NO, N2O, and N2. Because these products of nitrate respiration are all gaseous, they can easily be released to atmosphere and because of this the process is called denitrification (Fig. 24.11).
The process of denitrification is detrimental for agricultural purposes because N2 produced during it is much less readily available to organisms as a source of nitrogen. Denitrification is beneficial for sewage treatment because it converts amount of available nitrogen in the form of NO–3 to N2 thus effectively decreasing the amount of available nitrogen in the sewage treatment effluent that can stimulate algal growth.
The biochemical mechanism of nitrate (NO–3) respiration or dissimilative metabolism of nitrate (nitrate reduction) has been well studied in microorganisms like Escherichia coli, Paracoccus denitrificans and Pseudomonas stutzeri. In Escherichia coli the NO–3 is reduced only to NO–2 (Fig. 24.12) through its electron transport chain in which NADH acts as electron donor and NO–3 as electron acceptor.
In Paracoccus denitrificans and Pseudomonas stutzeri, where true denitrification occurs, the nitrate (NO–3) is reduced to nitrogen oxides (NO–2, NO, N2O) by a series of enzymes including nitrite reductase, nitric oxide reductase, and nitrous oxide reductase (Fig. 24.13).
During electron transport in both cases, a proton force is established across the plasma membrane and ATP is synthesized by ATPase enzyme in usual fashion. Additional ATP generates in case of P. denitrificans and P. stutzeri when NO–3 is reduced to dinitrogen (N2) because the nitrous oxide (NO) reductase is linked to the extrusion of proton.
Sulphate (SO2-4) Respiration (Sulphate Reduction):
Sulphate (SO2-4), the most oxidised form of sulphur, is a much less favoured electron acceptor than either O2 or NO–3. It is one of the major anions in sea water and is used by the sulphate-reducing bacteria in their dissimilative metabolism. The end product of sulphate respiration (sulphate reduction) is H2S, an important natural product that participates in many biogeochemical processes.
However, a large variety of sulphate- reducing bacteria are known. Desulfovibrio, Desulfomonas, Desulfotomaculum, and Desulfobulbus utilise lactate, pyruvate, ethanol, or certain fatty acids as electron donors reducing sulphate to H2S.
Desulfovibrio is the best-studied genus and commonly occurs in aquatic habitats or water logged soils containing abundant organic material and sufficient levels of sulphate. Growth and reduction of sulphate by Desulfotomaculum in certain canned foods leads to a type of spoilage called ‘sulphide stinker’.
In biochemical mechanism, the dissimilative sulphate reduction to H2S is an eight electron reduction process and proceeds through a number of intermediate stages (Fig. 24.14). The sulphate (SO2-4) is stable and requires activation before reduction.
The activation of sulphate takes place by means of ATP; the enzyme ATP sulfurylase catalyses the reaction resulting in the formation of adinosine 5′- phosphosulphate (APS).
The sulphate moiety of APS is reduced directly to sulphite (SO2-3) by the enzyme APS reductase with the release of Adenosine monophosphate (AMP). Once sulphite is formed it is finally reduced to H2S with the involvement of enzyme sulphite reductase.
The electron transport chain of and energy conservation (ATP synthesis) in sulphate reducing bacteria is shown in Figure 24.15. Electron transport reactions generate proton motive force that drives ATP synthesis by enzyme ATPase as usual.
Cytochrome C3, a periplasmic low potential cytochrome, accepts electrons from periplasmically located hydrogenase (H2ase) enzyme and transfers these electrons to a membrane-bound protein complex (HMC) that carries them across the plasma membrane thus making them available to APS reductase (for reduction of APS to SO2-3) and sulphite reductase (for reduction of SO2-3 to H2S). APS reductase and sulphite reductase are cytoplasmic enzymes.
Carbonate (CO2) Respiration:
Two major groups of obligate anaerobic prokaryotes (homoacetogens and methanogens) use CO2 as an electron acceptor and hydrogen (H2) as a major electron donor for energy conservation during anaerobic respiration. Homoacetogens and methanogens carry out the processes of acetogenesis and methanogenesis, respectively, and an overview of these processes is shown in Figure 24.16.
Acetogenesis generates ion gradients of H+ (proton motive force) or Na+ (sodium motive force) while methanogenesis generates ion gradients of H+ (proton motive force) across the plasma membrane during electron transport. These forces drive ATP synthesis via ATPase. Acetogenesis also involves ATP synthesis via substrate-level phosphorylation.
1. Acetogenesis:
Acetogenesis is carried out by homoacetogens (Acetoanaerobium noterae, Acetobactericum woodii, Clostridium aceticum, Clostridium thermoaceticum, Clostridium formicoacetium, and many others).
These bacteria reduce CO2 to acetate by acetyl-CoA pathway involving two linear pathways—one molecule of CO2 is reduced to methyl group of acetate, and the other molecule of CO2 is reduced to the carbonyl group of acetate—followed by their assembly at the end to form acetyl-CoA, which then is converted to acetate (Fig. 24.17).
In acetyl-CoA pathway of CO2 reduction to acetate, the ATP is synthesized when a sodium (Na+) gradient is established generating sodium motive force across the plasma membrane. The energized state of plasma membrane allows for ATP synthesis via the action of a Na+-powered ATPase enzyme. Additional ATP is also synthesized via substrate-level phosphorylation during the conversion of acetyl-CoA to acetate via acetyl-phosphate.
2. Methanogenesis:
Methanogenesis (methane production) is characteristic to a group of obligate anaerobic archaea (archaebacteria) called the methanogens.