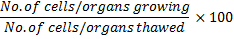Some of the glassware instruments used in microbiology laboratory are:- 1. Test Tubes 2. Test Tubes Racks 3. Test Tube Holder 4. Funnel 5. Volumetric Glassware 6. Non-Volumetric Glassware.
Also learn about the process of handling and caring of laboratory glasswares.
1. Test Tubes:
They are used to heat on hold reagents for observing chemical reaction.
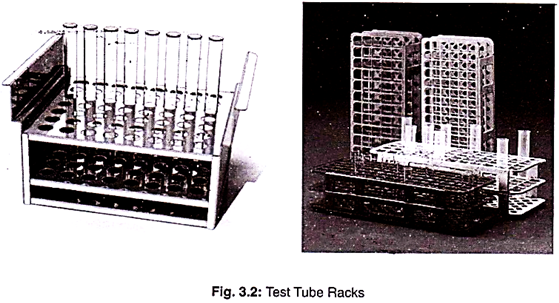
2. Test Tubes Racks:
It is used to hold the test tube in the upright position. These are made of metal or plastics.
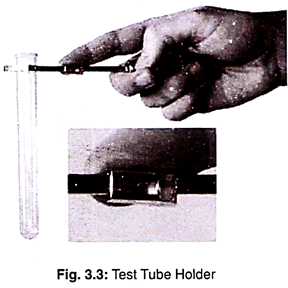
3. Test Tube Holder:
It is used during heating of the test tube.
4. Funnel:
It is used during filtration.
5. Volumetric Glassware:
It includes cylinders, pipettes, burette & volumetric flasks. It is used in measuring accurate volume of a liquid.
(a) Dropping Bottle:
They permit the fluid to flow in a drop wise fashion.
6. Non-Volumetric Glassware:
(a) Beaker:
It is used for heating liquid and for preparing reagent solution.
(b) Petri Dishes:
It is used for the aerobic culture of microbes.
(c) Stirring Rod:
It is used for dissolving the solute in preparing solution.
Handling and Caring of Laboratory Glasswares:
Care of Glassware:
While working in a laboratory the technician must get acquainted with the types of glasswares handled in the laboratory & use them appropriately. Improper use of glassware may lead to breakage.
There are basically 2 types of glassware:
(1) Borosilicate glassware
(2) Soda-lime glassware
Borosilicate is heat and chemical resistant. It can also stand mechanical stress & will not break due to sudden change of temperature. While soda-lime glass which is less resistant to mechanical shock and thermal shock. It is cheaper than borosilicate. It is easy to bend by heat.
Handling of Glassware:
Laboratory glassware is expensive proper care and handling reduces the risk of personal injury.
Some common type for the care and handling are:
1. Never leave the glass wares in the absence of attendant when it is heated, it will crack and explode.
2. Avoid scratching of glass in its daily use working the missing of a sonly in a beaker.
3. Use heat resistant glass while preparing solution of acids and alkaline.
Cleaning of Glass Wares:
New:
1. Soak in 2% HCl for overnight to neutralize any alkali present.
2. Wash in running tap water.
3. Boil in any synthetic detergent for 30 minutes. Rinse well with tap water and finally in distilled water.
Used Glassware’s:
Boil in a detergent for 30 minutes, clean thoroughly with a brush, rinse in tap water. Dry them in the hot air oven with the temperature not exceeding 80°C.
Potassium dichromate (kMno4) cleaning solution composition
kMno4 – 25gm
Water – 25ml
Conc. H2So4 – 50ml
Preparation:
Dissolve 25gm. Potassium dichromate in 25ml of water. Add 50ml of Conc. Sulphuric acid (slowly, always add acid to water) and cool store in a stoppered bottle. Dissolve when it starts turning green.
Test Tubes:
1. Autoclave to remove infected material.
2. Boil in a detergent solution for 30 minutes. Clean with a brush.
3. Rinse in running water and finally with distilled water and place them in test tube rack upside down and dry them in an oven.
4. Cotton plugs them and sterilizes them in the hot air oven.
Pipettes:
1. Soak in cleaning solution for overnight
2. Wash in running tap water.
3. Rinse in distilled water.
4. Dry on suction pump using spirit or acetone.
5. To sterilize plug mouth piece with cotton wool, wrap in craft paper and sterilize in hot air oven for 1 hr. at 160°c.
Pasteur Pipette:
1. Soak in 3% Lysol for 1 hour.
2. Wash it same as test tubes.
Glass Slides:
New:
1. Boil in a detergent for 30 min.
2. Place in Dichromate solution for overnight.
3. Wash in running water.
4. Keep methylated spirit.
5. For using, take them out with a forceps and hold them only by the edge.
Used slides – Wash it same as used glassware
Infected – Infected slides should be autoclaved and clean as early as possible new reuse slides used for examination of acid fast bacilli or gram stain)
Infected Glassware:
Contaminated material may be disposed in paper or cardboard wrappers and incinerated. Autoclave the glassware that has been contaminated. Having autoclaved, wash and prepare in the usual way.
Syringes:
Complete bacterial sterility can be achieved either by sterilizing them in the hot air oven or in an autoclave. Keep injection syringes separate from blood withdrawing syringes. Fresh syringes (sterilized) should be used for withdrawing blood for each patient. Before reusing they properly sterilize them. Needles used should be sharp and not with blunt ends.
Choice of Syringes and Needles:
All glass syringes are preferred over glass and metal ones. Preferably keep size 5ml or more syringes for withdrawing blood. Needles should be of size less than 21 (SWG). A needle with a smaller diameter would cause lysis of blood when used for blood withdrawing.
With-drawing needles should be at least an inch long.
New Syringes:
These are washed in the usual way then dried with acetone. Wrap the plunger and the barrel in a paper and sterilize in hot air oven.
Used Syringes:
Immediately after use, wash them thoroughly with cold water (hot water will coagulate proteins and will make the syringes difficult to clean). Clean then thoroughly in a detergent, brush the barrel properly, rinse in tap water and then in distilled water. Rinse in acetone and let dry. Sterilize in hot air oven as mentioned above.
Infected Syringes:
These should be washed at first with cold 2% Lysol solution and then clean as above. Syringes infected with highly virulent material should first be autoclaved. The syringes should be placed in the cold oven and be heated at 160°C for 90 minutes. Syringes not used for 3 months should be avoided and the barrel and the plunger should be sterilized separately (kept in a wrapper) by autoclaving them.
Needles:
(i) Should first be rinsed in cold water.
(ii) Clean the mounts with a cotton wool swab.
(iii) Wash again, rinse in acetone.
(iv) Pass a styllete through the hole to remove any plugs if present it is important to discard all needles with blunt tips, a hand lens can be used to examine needle tips).
(v) Serum hepatitis can be transmitted through using imperfectly cleaned and sterilized needles.
(vi) The needles should be sterilized in a hot air oven.
Disinfection of syringes by boiling.
In an emergency, syringes can be fairly effectively sterilized by boiling then in distilled water for at least 5 minutes after having cleaned them in the usual way.