In this article we will discuss about the Metabolism of Pyrimidine Nucleotides:- 1. Biosynthesis of Pyrimidine 2. Catabolism of Pyrimidine 3. Clinical Orientation.
Biosynthesis of Pyrimidine:
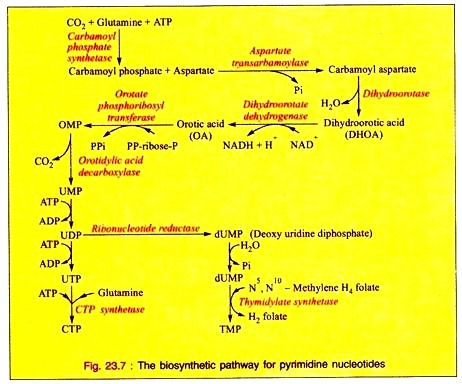
i. The synthesis of pyrimidine ring starts with the formation of carbamoyl phosphate from glutamine, ATP and CO2 being catalyzed by carbamoyl phosphate synthetase, present in the cytosol of the cell.
ii. Carbamoyl phosphate then reacts with aspartate by aspartate transcarbamoylase to form carbamoyl aspartate which, with the loss of water, is converted into dihydroorotic acid (DHOA) by the enzyme dihydroorotase.
iii. Dihydroorotic acid on dehydrogenation by dihydroorotate dehydrogenase utilizing NAD as coenzyme is converted into orotic acid (OA) which is by the action of orotate phosphoribosyl transferase, converted into orotidine monophosphate (OMP). This on subsequent decarboxylation by orotidylic acid decarboxylase forms uridine monophosphate (UMP).
iv. By further phosphorylation, UMP is converted into UDP and then to UTP.
v. UTP is converted into CTP in the reaction catalyzed by CTP synthetase utilizing ATP and glutamine.
vi. The enzyme Ribonucleotide reductase converts UDP into deoxyuridine diphosphate (dUDP) which is converted into dUMP. This is by the action of thymidylate synthetase with N5, N10-methylene H4 folate, converted into thymidine monophosphate (TMP).
The overall reaction is given in Fig. 23.7.
Catabolism of Pyrimidine:
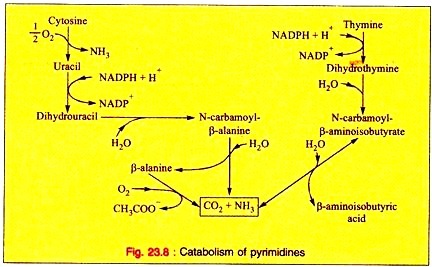
i. Liver is the main site for the catabolism of pyrimidine’s.
ii. CO2 is released from the pyrimidine nucleus representing a major pathway for the catabolism of uracil, cytosine, and thymine.
iii. The major end products of cytosine, uracil and thymine are β-alanine and β-aminoisobutyric acid, respectively.
iv. Thymine is the precursor of β-aminoisobutyric acid in humans and in animals. β-aminoisobutyric acid is excreted more in leukemia. This is due to increased destruction of cells and their DNA.
v. The β-aminoisobyutyric acid is converted into methylmalonic semi-aldehyde and then to propionate which turns to succinate.
The overall reactions for degradation is noted (Fig. 23.8).
Clinical Orientation of Pyrimidine:
i. The dietary purine and pyrimidine bases are not incorporated into tissue nucleic acids of humans. Rather, humans biosynthesize the purines and pyrimidine’s of tissue nucleic acids, ATP, and NAD+. CoA, etc. from amphibolic intermediates.
ii. The injected purine or pyrimidine analogs including anticancer drugs may be incorporated into DNA.
iii. The abnormal human diseases in purine metabolism include gout, Lesch-Nyhan syndrome, adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency. These diseases should be treated properly for eliminating the sufferings.
iv. The diseases of pyrimidine biosynthesis, although rare, include orotic aciduria.
v. The clinically significant disorders of pyrimidine catabolism are few, since the products of pyrimidine catabolism are highly soluble (CO2, HN3, and β-aminoisobuty rate).