The existence of lysosomes was subtly suggested for the first time in the early 1950s by a series of experiments carried out by Nobel Prize Laureate Christian de Duve and his co-workers.
These experiments were designed to identify the cellular locus of the two enzymes glucose-6-phosphatase and acid phosphatase.
Liver tissue homogenates were separated into nuclear, mitochondrial, microsomal, and cytosol fractions by differential centrifugation and enzyme assays were performed on each of the collected fractions.
Although results with glucose-6- phosphatase clearly indicated that this enzyme was bound to particles sedimenting with the microsome fraction, observations on the distribution of add, phosphatase were at first rather confusing.
The confusion centered around three seemingly peculiar but nonetheless reproducible findings:
(1) The acid phosphatase activities of tissue homogenates prepared for centrifugation using a glass tube and close-fitting plunger (Dounce homogenizer) were about one-tenth the value observed when tissue was more vigorously dispersed using a Waring blender;
(2) The total (i.e., combined) enzyme activity of the isolated centrifugal fractions analyzed following differential centrifugation was about twice the activity of the original homogenate; and
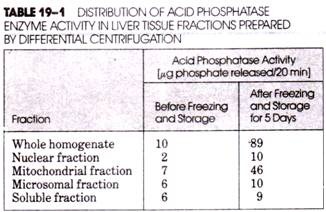
(3) After storage for several days in a freezer, both the enzyme activity of the homogenate and the collected fractions, especially the mitochondrial fraction, increased dramatically (Table 19-1).
These observations were explained when de Duve and his colleagues showed that acid phosphatase activity was confined to sedimentable particles, the surrounding membranes of which limited the accessibility of the substrate (β-glycerophosphate) used in the enzyme assay. Only when these membranes were disrupted and the acid phosphatase released from the particles was the enzyme activity demonstrable.
This occurred during vigorous dispersion in the Waring blender, which disrupted virtually all particles present. In contrast, only about 10% of the acid phosphatase-containing particles were disrupted by homogenization with the Dounce homogenizer, thus accounting for the low activity of the enzyme in the homogenate (Table 19-1). Some additional enzyme activity was released during and following centrifugal fractionation, but much larger quantities of enzyme were released by the membrane disruption that occurred during freezing and thawing.
At first, de Duve and his co-workers did not recognize that the acid phosphatase activity was associated with a distinct population of cellular particles. Instead, on the basis of the observed “latent” activity of the mitochondrial fraction obtained by differential centrifugation (compare the values before and after freezing in Table 19-1), de Duve believed that acid phosphatase resided within the mitochondria.
However, continued studies during the early 1950s in which the mitochondrial fraction was further divided centrifugally into a number of subtractions revealed that acid phosphatase was absent from fractions containing rapidly sedimenting mitochondria but was present in high concentrations in fractions containing slowly sedimenting mitochondria.
This observation, together with a newly developed appreciation of the potential contamination of sediments occurring during differential centrifugation, led de Duve to suspect that the acid phosphatase might, in fact, be associated with a special class of particles distinct from the mitochondria.
Added credence was given to this idea by the finding that four other acid hydrolases, namely, β-glucuronidase, cathepsin, acid ribonuclease, and acid deoxyribonuclease, were distributed through the centrifugal fractions in an identical manner. Thus, five hydrolytic enzymes, each having an acid pH optimum and acting on completely different substrates, appeared to be present in the same subcellular particle.
On the basis of the lytic effects of all of these enzymes, de Duve named the particles “lysosomes.” A number of additional enzymes have subsequently been identified in lysosomes (Table 19-2). Most of the chemical substances present in cells, including proteins, polysaccharides, nucleic acids, and lipids, are degraded by these enzymes.
It is interesting to note that the initial postulation of the existence of lysosomes was made by de Duve purely on biochemical grounds. However, in 1955, A. Novikoff, working with de Duve, examined centrifugal fractions rich in acid phosphatase activity using transmission electron microscopy and provided the first morphological evidence supporting the existence of these particles. The lysosomes were identified as small, dense, membrane-enclosed particles distinct from the mitochondria.
In recent years, sophisticated centrifugal methods have been devised for obtaining preparations that are rich in lysosomes. Nearly all preparations obtained by differential centrifugation are contaminated with quantities of mitochondria. Although the average sedimentation coefficient of mitochondria is greater than that of lysosomes, mitochondria are polydisperse with respect to size, so that the smaller mitochondria invariably sediment with the lysosomes.
Moreover, in tissues containing peroxisomes, the range of sedimentation coefficients for these organelles is almost identical to that of the lysosomes. Consequently, it is virtually impossible to obtain lysosome preparations that do not also contain peroxisomes. Somewhat greater success is obtained when isopycnic density gradient centrifugation is used in the last stages of the isolation procedure, for the equilibrium densities of lysosomes (1.22 g/cm3), mitochondria (1.19 g/cm3), and peroxisomes (1.23-1.25 g/cm3) in sucrose density gradients are slightly different.
Most density gradient procedures used to prepare lysosomes are modifications of the technique developed by W. C. Schneider (Fig. 19-1). Using this technique, most of the mitochondria are banded isopycnically at a density of about 1.19 g/cm3, whereas most of the lysosomes form a separate zone at about 1.22 g/cm3 and can be recovered independently from the density gradient.
By far the greatest purity of lysosomes is obtained from tissues of animals previously treated with Triton WR-1339 (a polyethylene glycol derivative of polymerized p-tert-octyl phenol), Dextran (a polymer of glucose), and Thorotrast (colloidal thorium hydroxide). These compounds are rapidly incorporated in large quantities by the cell’s lysosomes, significantly altering their density. For example, the incorporation of Triton WR-1339 reduces the average density of the lysosome from 1.22 to about 1.10 g/cm3.
It is interesting to note that although the density of lysosomes incorporating Triton WR-1339 is significantly reduced, their size is increased; the result is that Triton WR- 1339-loaded lysosomes have the same sedimentation coefficient as normal lysosomes but have a lower density.
The latent enzymatic effect originally noted by de Duve and his co-workers is still employed as a major criterion in evaluating the effectiveness of any lysosome isolation. Accordingly, the lysosome preparation is incubated under the appropriate conditions with the hydrolase substrate before and after treatments known to disrupt the lysosome membranes.
If the original preparation contains intact lysosomes, then no substrate is hydrolyzed before treatment (most substrates of the lysosomal hydrolases are unable to permeate the lysosome’s membrane); however, disruption of the membrane (by sonification, repeated freezing and thawing, addition of lytic agents such as bile salts, digitonin, Triton X-100, etc.) and release of the lysosomal enzymes are quickly followed by hydrolysis of the added substrates.
Structure and Forms of Lysosomes:
Lysosomes are a structurally heterogeneous group of organelles and vary dramatically in size and morphology. As a result, it is difficult to identify lysosomes strictly on the basis of morphological criteria. When lysosome-rich fractions were initially isolated centrifugally by de Duve and Novikoff and examined with the electron microscope, it was found that the suspected lysosomes were generally about the same size as small mitochondria.
Typically, they varied in diameter from about 0.1 to 0.8 μm, were bounded by a single membrane, and were usually somewhat electron-dense. Identification of lysosomes in sections of whole cells is considerably more difficult because other small, dense organelles are also bounded by a single membrane.
The application of cytochemical procedures at the level of the electron microscope in which the lysosomes are identified on the basis of their enzyme content is much more reliable. Notable among such procedures is that introduced in 1952 by G. Gomori and that is routinely employed in variously modified forms for the identification of lysosomes on the basis of their high acid phosphatase content.
In the Gomori method, the tissue to be examined is incubated at pH 5.0 in a medium containing β-glycerophosphate (a substrate for acid phosphatase) and a lead salt (such as lead nitrate). Phosphate enzymatically cleaved from the substrate during incubation combines with the lead ions to form insoluble lead phosphate, which precipitates at the locus of enzyme activity.
Because the lead phosphate is electron-dense, electron microscopy reveals the lysosomes as dark, granular organelles (Fig. 19- 2). For identification with the light microscope, ammonium sulfide may be used to convert the lead phosphate to lead sulfide, which appears black. The Gomori reaction may be carried out with fixed and sectioned material, as well as with fresh tissue, albeit with reduced efficiency as a result of some enzyme in- activation during and following fixation.
Several different lysosomal forms have been identified within individual cells, including:
(1) Primary lysosomes,
(2) Secondary lysosomes, and
(3) Residual bodies.
Primary Lysosomes:
Primary lysosomes, or proto- lysosomes, are newly produced organelles bounded by a single membrane and believed to be derived from the trans face of the Golgi apparatus. Although varying somewhat in size, primary lysosomes are typically about 100 nm in diameter. Primary lysosomes are virgin particles in that their digestive enzymes have not yet taken part in hydrolysis.
Secondary Lysosomes:
Two different kinds of secondary lysosomes can be identified: heterophagic vacuoles (also called heterolysosomes or phagolysosomes) and autophagic vacuoles (also called auto- lysosomes). Heterophagic vacuoles are formed by the fusion (see below) of primary lysosomes with cytoplasmic vacuoles containing extracellular substances brought into the cell by any of a variety of endocytic processes. Following fusion, the hydrolases of the primary lysosomes are released into the vacuole (variously called a phagosome or endosome). Autophagic vacuoles contain particles isolated from the cell’s own cytoplasm, including mitochondria, microbodies, and smooth and rough fragments of the endoplasmic reticulum.
The autodigestion of cellular organelles is a normal event during cell growth and repair and is especially prevalent in differentiating and dedifferentiating tissues and tissues under stress. Autophagic vacuoles containing partially degraded mitochondria are shown in Figure 19-3.
The formation of heterophagic and autophagic vacuoles is soon followed by enzymatic digestion of the vacuolar contents. As digestion proceeds, it becomes increasingly difficult to identify the nature of the original secondary lysosome and the more general term digestive vacuole is used to describe the organelle at this stage.
Residual Bodies:
Endocytosed substances and parts of autophagocytosed organelles that are not digested within the secondary lysosomes and transferred to the cytoplasm are retained (usually temporarily) within the vacuoles as residues.
Lysosomes containing such residues are called residual bodies (sometimes also called telolysosomes or dense bodies). Residual bodies are large, irregular in shape, and are usually quite electron-dense. The undigested residues often take the form of whorls of membranes, grains, amorphous masses, ferritinlike or hemosiderinlike particles, or myelin figures (Fig. 19-4). Residual bodies often fail to display the degree of hydrolytic activity associated with the primary and secondary lysosomes.
Formation and Function of Lysosomes (the “Vacuolar System”):
Lysosomal enzymes are concerned with the degradation of metabolites and not with cellular synthetic or transfer reactions. On the basis of numerous cytochemical observations, it is generally believed that primary lysosomes are formed by budding from the trans face of the Golgi apparatus. The primary lysosomes are dispatched as either smooth or coated vesicles having a diameter of about 50-100 nm. After budding, the coat is lost. The acid hydrolases that are destined for lysosomes are synthesized by ribosomes of the rough ER in the vicinity of the Golgi bodies.
Some of these hydrolases are discharged into the lumenal phase of the ER and others remain anchored in the ER membranes. Through either the dispatchment of tiny vesicles from the ER or via direct communication through cisternae, the hydrolases make their way to the cis face of the Golgi body. After purification and processing in successive Golgi cisternae, the hydrolases are released from the trans face of the Golgi apparatus in the form of primary lysosomes.
This concept, depicted diagrammatically in Figure 19-5, is supported by a large number of observations made with a variety of tissues. The process is reminiscent of the formation of zymogen granules for secretion by Golgi bodies. Because of the intimate association between Golgi bodies, ER, and primary lysosomes, regions of cells containing these organelles are sometimes called “GERL” (i.e., “Golgi-Endoplasmic Reticulum-Lysosome”) complexes.
The synthesis of the lysosomal enzymes involves the preliminary assembly of a signal sequence that directs the large ribosomal subunit to its appropriate docking site on the endoplasmic reticulum. Following this, the elongating polypeptide is discharged through the membrane into the intracisternal space. Virtually all of the lysosomal enzymes are glycoproteins. Core glycosylation occurs during translation but final processing occurs in the cis Golgi cisterna.
Using histochemical procedures it is possible to localize lysosomal enzyme activity in the Golgi apparatus; this, of course, supports the view that lysosomal enzymes progressively transit through at least some of the Golgi cisternae. The carbohydrate portions of the lysosomal enzymes include an uncommon mannose-6-phosphate-containing oligosaccharide. Recent studies indicate that the Golgi membranes contain a specific receptor for mannose-6-phosphate and it is believed that these receptors play an important role in directing the lysosomal hydrolases into newly forming primary lysosomes.
Heterophagy:
Extracellular materials brought into the cell by endocytosis are enclosed within vacuoles called endosomes. These materials may later be rejected unaltered by exocytosis, or the endosome may fuse with one or more primary lysosomes that empty their digestive hydrolases into the newly formed particle (now called a secondary lysosome, see Fig. 19-5). Lysosomal digestion of endocytosed material is termed heterophagy.
The fusion of primary lysosomes with endosomes has been demonstrated in vivo in a number of tissues using various exogenous markers introduced into the organism. These markers, which include horseradish peroxidase, ferritin, and hemoglobin, are engulfed by the tissue cells and are later detected within secondary lysosomes along with lysosomal hydrolases.
Z. A. Cohn and B. Benson employed 3H-labeled leucine to trace the fate of newly synthesized hydrolases in peritoneal phagocytes of mice. They found that pinocytic activity was greatly increased when these cells were incubated in blood serum. Autoradiographic analysis revealed that the labeled hydrolases appeared first in the Golgi region of the cells and later within pinocytic vesicles or endosomes.
Their observations support the proposal that secondary lysosomes are formed by the fusion of endosomes and primary lysosomes. Moreover, Cohn and Benson also found that the rate at which hydrolases were produced by the cells was related to the level of pinocytic activity, suggesting that the production of primary lysosomes may somehow be regulated by endocytosis (see below).
In some cells, several small primary lysosomes may fuse with a single large endosome; in other cells, large primary lysosomes sequentially fuse with a number of small endosomes.
The contents of the secondary lysosome change dramatically with time as:
(1) The contents of the lysosome are enzymatically degraded,
(2) New materials are introduced through fusion of additional endosomes, and
(3) Additional hydrolases are added by the fusion of new primary lysosomes.
The hydrolases in the secondary lysosome break down the endocytosed materials, producing a variety of useful substances (e.g., amino acids, sugars, etc.) as well as some useless waste products. It is generally agreed that usable materials make their way across the membrane of the secondary lysosome and enter the cytosol, where they participate in cellular metabolism.
This transfer probably takes the form of passive diffusion or facilitated or active transport. Eventually, digestion and absorption are terminated) leaving only residues and denatured enzymes within the vacuole, which is now referred to as a residual body. In many cells, residual bodies fuse with the plasma membrane, and this is followed by exocytosis (Fig. 19-5).
In some ceils (especially those of higher organisms), residual bodies accumulate within the cytoplasm or continue to increase in size, eventually interfering with the normal activities of the cell and resulting in cell death. Progressive lysosome engorgement is believed to be involved in the aging process.
Autophagy:
The isolation and digestion of portions of a cell’s own cytoplasmic constituents by its lysosomes occurs in normal cells and is termed autophagy (Fig. 19-5). The phenomenon is most dramatic in the tissues of organs undergoing regression (e.g., changes in the uterus following delivery, during metamorphosis in insects, etc.).
Autophagic vacuoles containing partially degraded mitochondria, smooth and rough endoplasmic reticulum, micro-bodies, glycogen particles, or other cytoplasmic structures are frequently observed in tissue sections examined with the electron microscope. Cellular autophagy results in a continuous turnover of mitochondria in liver tissue. The half-life of the liver mitochondrion is about 10 days and corresponds to the destruction of one mitochondrion per liver cell every 15 minutes.
Distribution of Lysosomes:
Since their initial discovery in mammalian liver, lysosomes have been identified in many different cells and tissues; some of these are listed in Table 19-3. The greatest variety of tissues found to contain lysosomes occurs in animals. Although most studies have been carried out using mammalian tissues, lysosomes have been identified in insects, marine invertebrates, fish, amphibians, reptiles, and birds.
Lysosomes are particularly numerous in epithelial cells of absorptive, secretory, and excretory organs (liver, kidneys, etc.). They are also present in large numbers in the epithelial cells of the intestines, lungs, and uterus. Phagocytic cells and cells of the reticuloendothelial system (e.g., bone marrow, spleen, and liver) have also been found to contain large numbers of lysosomes. Few lysosomes occur in muscle cells or in acinar cells of the pancreas. Lysosomes are produced by certain cells in tissue culture (HeLa cells, monocytes, lymphoctyes, etc.). Although it has a number of functions not shared by lysosomes of animal cells, the large vacuole of many plant cells is a modified lysosome. Some of the various roles played by the lysosomes are summarized in Table 19-4.
Leukocytes, especially granulocytes, are a particularly rich source of lysosomes, and this is related to their physiological role as scavengers of microorganisms or other foreign particles in the blood. Following phagocytosis of a bacterium by a leukocyte, numerous lysosomes fuse with the endocytic vacuole containing the microorganism and initiate its digestion. The lysosomes of granular leukocytes are especially large and readily visible by light microscopy. Once the lysosome content of the leukocyte is exhausted, the white blood cell dies.
Plant Vacuoles:
Many plant cells contain one or more vacuoles, which possess some of the properties of lysosomes. In immature and actively dividing plant cells the vacuoles are quite small. As the cells mature, the vacuoles coalesce to form larger compartments. Mature cells of higher plants usually have a larger, central vacuole that may occupy as much as (occasionally more than) 80% of the total cell volume.
The membrane enclosing the plant cell vacuole is called the tonoplast. Like lysosomes, plant cell vacuoles contain hydrolytic enzymes. In addition, they usually contain sugars, salts, acids, and nitrogenous compounds such as alkaloids and anthrocyanin pigments. The pH of the plant vacuole may be as high as 9 or 10 due to large quantities of alkaline substances or as low as 3 due to the accumulation of quantities of acids (e.g., citric, oxalic, and tartaric acids).
The plant vacuole is the major contributor to the turgor that provides support for the individual plant cell and contributes to the rigidity of the leaves and younger parts of the plant. Water accumulation in the vacuole as a result of the osmotic effects of the dissolved substances causes the vacuole to expand, pushing outward against the cytoplasm and cell wall. When there is a lack of water the turgor diminishes and these results in wilting.
Lysosome Precursors in Bacteria:
Although bacterial cells do not possess lysosomes, they do contain a variety of hydrolases that are believed to be localized in the space between the plasma membrane and the cell wall. These hydrolases may be synthesized by ribosomes attached to the plasma membrane and then dispatched through it. The bacterial hydrolases play a digestive role, breaking down complex substrates in the cell’s environment and providing smaller molecules required for cell growth. Bacterial hydrolases also participate in sporulation and autolysis.
Although the latter process destroys the individual cells involved, it is highly beneficial to the bacterial population as a whole, for it provides for the survival of a small number of cells under unfavorable environmental conditions. In-folding of the bacterial membrane to form internalized extracellular pockets containing both hydrolases and their substrates would provide the “evolutionary link” with lysosomes of animal and plant cells.
Regulation of Lysosome Production:
The mechanism proposed for primary lysosome formation is strikingly similar to that proposed for zymogen granule formation in pancreatic cells and other instances of secretory protein synthesis. This similarity does not seem so unusual when one considers the following. The enzymatic contents of primary lysosomes are discharged into vacuoles (i.e., endosomes) that are derived from the plasma membrane and that contain extracellular materials. Consequently, the mechanism is similar to secretion except that the extracellular space into which the secretory products pass is internalized.
In secretory cells, the production of new secretory products is regulated by a feedback mechanism in which secretion itself acts as a stimulus for the production of additional secretory materials. The experiments of Cohn and Benson described above demonstrate the relationship that exists between endocytic activity and lysosomal enzyme synthesis. It has therefore been suggested that the passage of endocytic vesicles into the Golgi regions of the cell is followed by the discharge of some primary lysosomes and that this triggers the synthesis of new acid hydrolases.
Disposition and Action of the Lysosomal Hydrolases:
Many of the lysosome’s enzymes are released into the surrounding environment when these organelles are physically or chemically disrupted. Those enzymes that are so readily solubilized are believed to be located in the interior of the organelle. Other lysosomal hydrolases cannot be solubilized or are extracted with great difficulty and are thought to be an integral part of the lysosome membrane together with other proteins and lipids. Some of the enzymes known to be present in lysosomes are listed in Table 19-2; it is to be noted that while this list is extensive, it is by no means complete.
All the substrates of lysosomal enzymes are either polymers or complex compounds and include proteins, DNA, RNA, polysaccharides, carbohydrate side chains of glycoproteins and glycolipids, lipids, and phosphates. The lysosomal breakdown of proteins into amino acids illustrates how these enzymes act in concert. The initial hydrolysis of protein is effected by cathepsins D and E and also by collagenase. These enzymes cleave peptide bonds and produce peptide fragments of varying length.
The peptides, together with previously undigested proteins, are further hydrolyzed to individual amino acids by cathepsins A and B. Cathepsin C, arylamidase, and the lysosomal dipeptidases act on specific peptides, producing additional amino acids.
The breakdown of DNA and RNA is initiated by the enzymes acid deoxyribonuclease and acid ribonuclease. The resulting oligonucleotides are then degraded first by phosphodiesterase and then by acid phosphatase, producing nucleosides and inorganic phosphate. Lysosomes also possess all the enzymes necessary for hydrolysis of lipids and polysaccharides.
Some lysosomal enzymes are part of the membrane encasing the organelle. Among the enzymes found to be integral parts of the lysosome membrane are acetylglucosaminidase, glucosidase, and sialidase. Arylsulfatase, acid phosphatase, ribo- nuclease, and glucuronidase may also be bound to the membrane under certain conditions.
Enzymes freed from disrupted lysosomes exhibit a wide variation in stability. Some are particularly resistant to autolysis and retain their activity for months when appropriately refrigerated; others lose their activity only a few hours following tissue disruption. Of the lysosomal enzymes isolated and characterized to date, most are glycoproteins, including cathepsin C, acid deoxyribonuclease, glucuronidase, and acetylglucosaminidase.