In this article we will discuss about Echinoderms:- 1. Taxonomic Retrospect of Echinoderms 2. Definition and Origin of Echinoderms 3. Classification 4. Phylogenetic Considerations 5. Research 6. Habit and Habitat 7. Size 8. Shape and Symmetry 9. Body Wall and Skeleton 10. Digestive System 11. Coelom 12. Haemal System or Blood Lacunar System 13. Excretory System 14. Nervous System 15. Reproductive System and Others.
Contents:
- Taxonomic Retrospect of Echinoderms
- Definition and Origin of Echinoderms
- Classification of Echinoderms
- Phylogenetic Considerations of Echinoderms
- Research of Echinoderms of Echinoderms
- Habit and Habitat of Echinoderms
- Size of Echinoderms
- Shape and Symmetry of Echinoderms
- Body Wall and Skeleton of Echinoderms
- Digestive System of Echinoderms
- Coelom of Echinoderms
- Haemal System or Blood Lacunar System of Echinoderms
- Excretory System of Echinoderms
- Nervous System of Echinoderms
- Reproductive System of Echinoderms
- Regeneration of Echinoderms
- Development of Echinoderms
- Affinities of Echinoderms
1. Taxonomic Retrospect of Echinoderms:
1. The members of the phylum Echinodermata are known to mankind since ancient times. Echinoderms were first given the status of a distinct group by Bruguiere in 1791.
2. But Jacob Klein (1734) is credited with coining the name Echinodermata.
3. Linnaeus studied a number of Echinoderms, but placed Echinus, Asterias and Holothuria under Mollusca.
4. Lamarck (1801) included the Echinoderms and medusoid coelenterates under the class Radiata.
5. It was not until 1847 that Frey and Leuckart recognised the Echinoderms as a distinct taxon.
6. Huxley (1875) proposed a new name Deuterostomata and placed all the bilaterally symmetrical coelomates under the group.
7. The term Enterocoela including Chaetognatha, Echinodermata, Hemi- chordata and Chordata, was also used by many authors. The group Enterocoela seems to be synonymous with Deuterostomata. It is now established that the different groups under Deuterostomata or Enterocoela are widely separated for their structural diversities and there is no justification to include them under one group.
8. Metschnikoff (1881) included the Echinoderms and Hemichordates under Ambulacraria on the basis of striking resemblances between the Echinoderm larvae and Tornaria larva of Hemichordata.
9. In spite of the similarities between Echinoderms and Hemichordates, they are placed in separate groups.
10. The names of Bather (1900), Mortensen and Lieberkind (1928), Cuenot (1948), Hyman (1955), Moore (1966-78), Barnes (1987) are to be mentioned for their outstanding and extensive treatise on different Echinoderms.
2. Definition and Origin of Echinoderms:
Echinoderms are bilaterally symmetrical (in larval stage) or pentamerous radially symmetrical (in adults) having mesodermal calcareous ossicles as exoskeleton, enterocoelom, water-vascular system and without distinct head.
Origin:
Palaeontological record shows that all groups of modern echinoderms have their origin in early palaeozoic stock (Lower Cambrian) and predominate in Mesozoic and Caenozoic eras (R. C. Moore, 1977).
3. Characteristic Features of Echinoderms:
1. The echinoderms are exclusively marine, non-colonial and largely bottom dwellers.
2. The body exhibits radial and pentemerous symmetry in adult, but the larvae are bilaterally symmetrical.
3. Distinct anterior end or head is lacking.
4. The body is distinguishable into oral (bearing mouth) and aboral surfaces (surface away from the mouth).
5. The surface of the body is covered by calcareous ossicles, warts or spines, derived from mesodermal tissue.
6. The oral surface of the body is marked by five equidistant radiating ambulacra with intervening grooves, called interambulacra.
7. Mutable connective tissue is present.
8. A spacious coelom is present, developed as outgrowths of the archenteron, lined by ciliated peritoneum of mesodermal origin.
9. The coelom contains a well-developed, mostly coiled digestive tract.
10. A peculiar coelomic water vascular system (briefly called W.V.S.) or ambulacral system is present. Primitively the water vascular system probably functioned in collecting and transporting food, but now it performs many functions such as feeding, locomotion and respiratory, etc.
11. The nervous system is primitive and consists of radially arranged diffused nerve cords.
12. There are no distinct respiratory and excretory systems in most of the cases.
13. The blood vascular (haemal) system is present in all echinoderms. It becomes highly developed in Echinoids and Holothuroids.
14. Special sense organs are poorly developed.
15. The sexes are separate (mostly larger species) or hermaphrodites (smaller species).
16. Coelomic gonads.
17. Simple gonoducts.
18. Fertilization is usually external.
19. Cleavage is radial and indeterminate type of development.
20. The development of echinoderms involves a large number of bilaterally symmetrical, ciliated and free-swimming larval forms.
4. Classification of Echinoderms:
Classification in Outline:
The older classification is presented up, to sub-phylar rank on the mode of existence. So the known members of the phylum Echinodermata is divided into two subphyla— Eleutherozoa (non-sessile type) and Pelmatozoa (sessile types) which were in vogue for a long.
The Eleutherozoa includes four classes—Asteroidea, Ophiuroidea, Echinoidea and Holothuroidea, and Pelmatozoa includes only single living class—Crinoidea.
But H. B. Fell (1948, 1965), the authority on echinoderm taxonomy of Harvard University, USA, rejected the older classification as it was an artificial one because it was on the basis of mode of existence.
He proposed a classification on the basis of palaeontology and comparative morphology and divided into 4-subphyla which are based on more fundamental relationship providing four major patterns of body symmetry, related to the internal organs as well as the skeleton.
The scheme of classification presented in this book (4th ed.), largely based on the classification plan outlined by Edward E. Ruppert and Robert D. Barnes (1994, 6th ed.).
1. Subphylum Homalozoa:
e.g., Enoploura.
2. Subphylum Crinozoa:
Class Eocrinoidea
e.g., Mimocystites.
Class Cystidea
Order Rhombifera
e.g., Cystoblastus.
Order Diploporita
e.g., Proteroblastus.
Class Blastoidea
e.g., Codaster
Class Crinoidea
Subclass Inadunata
Order Flexibilia
e.g., Forbesiocrinus.
Order Camerata
e.g., Reteocrinus, Xenocrinus.
Subclass Articulata
Order Millericrinida
e.g., Hyocrinus, Calamocrinus.
Order Cyrtocrinida
e.g., Holopus.
Order Bourgueticrimda
e.g., Bathycrinus.
Order Isocrinida
e.g., Metacrinus.
Order Comatulida
e.g., Antedon, Florometra, Heliometra.
3. Subphylum Asterozoa:
Class Asteroidea
Order Platyasterida
e.g., Platyasterias, Luidia.
Order Paxillosida
e.g., Astropecten, Ctenodiscus.
Order Valvatida
e.g., Culcita, Goniaster, Linckib, Oreaster, Archaster.
Order Spinulosida
e.g., Asterina, Echinaster, Acanthaster, Crossaster, Pteraster.
Order Forcipulata
e.g., Asterias, Heliaster, Leptasterias, Brisinga, Zoroaster, Oidina.
Class Ophiuroidea:
Order Oegophiurida
Order Phrynophiurida
e.g., Ophiomyxa, Gorgonocephalus.
Order Ophiurida
e.g., Amphiura, Ophiura, Ophiocoma, Ophiothrix, Ophioderma.
Class Concentricycloida
e.g., Xyloplax
4. Subphylum Echinozoa:
Class Echinoidea
Subclass Perischoechinoidea
e.g., Bothriocidaris.
Order Cidaroidea
e.g., Eucidaris, Cidaris.
Subclass Euechinoidea
Superorder Diadematacea
Order Pedinoida
e.g., Caemopaedina.
Order Diadematoida
e.g., Diadema.
Order Echinothuroida
e.g., Asthenosoma
Superorder Echinacea
Order Arbacioida
e.g., Arbacia.
Order Temnopleuroida
e.g., Temnopleurus, Toxopneustes, Lytechinus.
Order Echinoida
e.g., Echinus, Paracentrotus, Strongylocentrotus, Echinometra.
Superorder Gnathostomata:
Order Holectypoida
e.g., Echinoneus.
Order Clypeasteroida
e.g., Clypeaster, Fibularia, Mellina, Rotula.
Superorder Atelostomata:
Order Spatangoida
e.g., Spatangus, Echinocardium, Meoma, Lovenia.
Order Cassiduloida
e.g., Echinolampus.
Class Holothuroidea:
Order Dactylochirotida
e.g., Echinocucumis.
Order Dendrochirotida
e.g., Cucumaria, Thyone.
Order Aspidochirotida
e.g., Holothuria, Actinopyga, Thelenota.
Order Elasipodida
e.g., Pelagothuria, Elpidia.
Order Molpadida
e.g., Molpadia, Caudina.
Order Apodida
e.g., Leptosynapta, Synapta, indicates fossil group.
Classification with Characters:
A. Subphylum Homalozoa (Mid- Cambrian —Devonian):
Features:
1. Extinct, irregular, palaeozoic.
2. Carpoids (resembling crinoids but laterally compressed giving the evidence of a bilateral symmetry).
Example:
Enoploura.
B. Subphylum Crinozoa:
Features:
1. Radially symmetrical echinoderms with a globoid or cup-shaped theca and brachioles or arms.
2. Mostly attached, with oral surface directed upward.
This subphylum includes the fossil eocrinoids, cystoids and the fossil and living crinoids.
1. Class Eocrinoidea (Early Cambrian to Ordovician):
Features:
1. The oldest extinct crinoids.
2. They were stalked or stalk-less, with an enclosed theca.
3. The upper or oral end contained five ambulacra and five to many brachioles.
Example:
Mimocystites.
2. Class Cystidea (Ordovician—Silurian):
Features:
1. The well-known group of extinct echinoderms.
2. They have vase-like bodies which remain fixed with the substratum directly or through a stalk.
3. The theca is composed of many rigid polygonal plates.
4. The plates constituting the theca are mostly porous and are perforated by canals.
5. The food-grooves extend over the surface of the theca.
6. The branchioles are devoid of pinnules.
(i) Order Rhombifera:
Features:
1. The theca canals produce various types of pores in a few or in all plates of the theca.
Examples:
Caryocystites, Echinosphaerites, Glyptocystites, Lovenicystis, Cystoblastus.
(ii) Order Diploporita:
Features:
1. The theca canals are usually in the form of diplopores in all or in some of the thecal plates.
Examples:
Proteroblastus, Aristocystites, Sphaeronites, Mesocystis, Asteroblastus.
(iii) Order Blastoidea (Ordovician—Permian):
Features:
1. The members of this extinct class had pentamerous radially symmetrical thecae consisting of thirteen plates in three rows.
2. They were mostly fixed forms and remained attached with the substratum directly or through a short stalk.
3. The ambulacra are petaloid and are five in number.
4. They possess the characteristic respiratory structures, called hydrospires.
Examples:
Codaster, Pentremites, Phaenoschisma, Orophocrinus, Troostocrinus, Zygocrinus.
3. Class Crinoidea (Cambrian—Recent):
[Gk. crinon = lily; eidos = form], Approx. 700 species.
Features:
1. Stalked and free-living echinoderms.
2. Body exhibits stong pentamerous symmetry.
3. Well-developed movable arms which are typically branched and bearing pinnules.
4. Oral surface is directed upwards.
5. Mouth is centrally placed and anus is generally excentrically placed on the oral surface of the body.
6. The theca (protective covering or case) on the aboral side is differentiated into a non-porous cup-like calyx.
7. Madreporite, spines and pedicellariae are wanting.
8. Barrel-shaped, free-swimming larva, called doliolaria larva, with 5 ciliated bands.
It includes sea lilies and feather stars.
1. Subclass Inadunata (Up. Cambrian— Permian):
Features:
1. Extinct, stalked crinoids with or without cirri.
2. The calyx is rigid and the ambulacra are mostly open.
3. The pinnules may or may not be present.
4. The lower arm ossicles are separated from the calyx.
Examples:
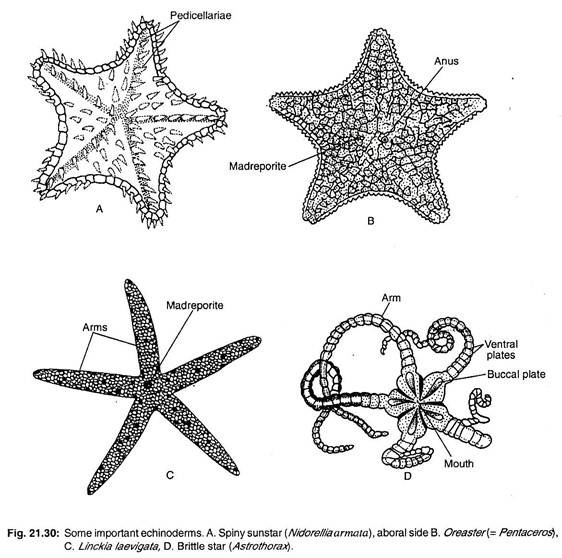
Anartiocrinus, Ottawacrinus, Botryocrinus (Fig. 21.30C), Hybocystitus.
2. Subclass Flexibilia (Ordovician— Permian):
Features:
1. Extinct crinoids.
2. The calyx is flexible and the ambulacra are covered.
3. The pinnules are totally absent.
4. The lower arm ossicles are united with the calyx.
Examples:
Forbesiocrinus.
3. Subclass Camerata (Ordovician— Permian):
Features:
1. Extinct crinoids.
2. The calyx is rigid with branched pinnulated arms.
3. The arm ossicles on the lower side are united with calyx.
4. The tegmen is armoured with plates and covers the mouth and ambulacra.
5. Usually an anal tube is present.
Examples:
Xenocrinus, Reteocrinus, Archaeocrinus, Platycrinus, Technocrinus.
4. Subclass Articulata:
Features:
1. The subclass contains both living and fossil crinoids with flexible pentamerous calyx.
2. The arm ossicles on the lower side are united with the calyx.
3. The arms are generally branched.
4. The tegmen is leathery with small plates.
5. The ambulacra and the mouth remain open.
It includes 5 orders:
(i) Order Millericrinida:
Stalked sea lilies without cirri.
Examples:
Hyocrinus, Ptilocrinus.
(ii) Order Cyrtocrinida:
The sea lilies of this order are attached to the substrate with a very short stalk.
Examples:
Holopus.
(iii) Order Bourgueticrinida (Sea lilies):
These are stalked small sea lilies and lack cirri. These lilies possess 5 or 10 very short arms.
Examples:
Rhizocrinus, Bathycrinus (Bathycrinus recorded in about 10,000 m in depth and is known in deepest depth).
(iv) Order Isocrinida (Sea lilies):
These sea lilies have long stalks with cirri. Many species are attached to the hard substrates.
Examples:
Cenocrinus, Metacrinus, Neocrinus, Isocrinus (Fig. 21.29D).
(v) Order Comatulida (Feather stars):
These are stalkless, unattached crinoids and called feather stars. Most of crinoid species are included with in this order.
1. Calyx upward.
2. A series of jointed, flexible appendages, called cirri, occur at the base of the body and help to grasp the solid substrates.
Examples:
Antedon (Fig. 21.29C), Comantheria, Florometra, Heliometra, Nemaster, Commisia.
C. Subphylum Asterozoa:
Features:
1. Radially symmetrical, free living echinoderms.
2. Body composed of a flattened central disc and radially arranged arms or rays.
3. Oral surface directed downward.
4. Anus, when present, is placed aborally.
The subphylum includes 3 classes:
1. Class Asteroidea (Cambrian—Present):
[Gk. aster = star; eidos = form]; Approx. 1800 species.
Features:
1. Body star-shaped.
2. Arms not sharply set off from the central disc.
3. Number of the arms usually 5 which may increase in some forms.
4. Ambulacral grooves open and contain rows of tube-feet.
5. Ambulacra (sing ambulacrum) restricted to the oral surface of the body.
6. Arms are hollow and each arm bears continuation of the coelom.
7. Madreporite situated on the aboral surface.
8. Pedicellariae is present.
9. The larval forms are bipinnaria and/or brachiolaria.
10. Generally called sea star.
The class is divided into 5 orders:
(i) Order Platyasterida:
Features:
1. Primitive, mostly extinct asterioids.
2. Aboral surface bears large upright spines beset with two or three circlets of spinelets.
3. Margin of the arm is formed by the infra-marginal plates.
4. No anus.
5. Tube-feet without suckers.
The two living genera are platyasterias and Luidia.
(ii) Order Paxillosida:
Features:
1. The tube-feet lack suckers.
2. The ampullae are bifurcated.
3. Sea stars with marginal plates and usually with paxillae (i.e., upright movable spines) on the ambulacral surface.
4. Pedicallariae either sesssile or pectinate types.
Examples:
Ctenodiscus, Goniopecten, Astropecten (Fig. 21.26), Dipsacaster, Culcita, Linckia (Fig. 21.30C).
(iii) Order Valvatida:
Features:
1. Tube-feet with terminal suckers.
2. Aboral plates usually flattened and have mosaic arrangement.
Examples:
Archaster, Asterina, Odontaster, Acodontaster, Notioceramus, Chitonaster, Oreaster (= Pentaceros, Fig. 21.30B), Porcellanaster, Morelha (Fig. 21.30A).
H. B. Fell used Phanerozonida instead of Paxillosida and Valvatida.
(iv) Order Spinulosida:
Features:
1. Marginal plates are sometimes conspicuous, but in general, plates are absent or small.
2. Spines are present on the aboral surface either singly or in groups.
3. Tube-feet are provided with suckers and occur in two rows in each ambulacral groove.
4. Pedicellariae rarely present.
5. Ampullae may be simple or bifurcated.
Examples:
Echinaster, Henricia, Ganerio, Cycethra, Patiria, Solaster (Fig. 21.27E), Acanthaster, Crossaster, Pteraster.
(v) Order Forcipulata:
Features:
1. Marginal plates are not prominet.
2. Spines occur singly.
3. Tube-feet are arranged in two or four rows and are provided with suckers.
4. Pedicellariae is stalked.
Examples:
Asterias (Fig. 21.29A), Odinia, Ordinella, Asterostephane, Heliaster, Leptasterias, Pisaster, Brisinga, Zoroaster (Fig. 21.27D).
2. Class Ophiuroidea (Carboniferous— Recent):
[Gk. ophis = snake; oura = tail; oidos = form]; Approx. 2100 species.
Features:
1. They are commonly termed the brittle stars or serpent stars.
2. Body pentamerous and star-shaped.
3. It has a distinct central disc with 5 elongated flexible arms.
4. The arms are sharply marked off from the central disc.
5. Ambulacral grooves absent excepting some fossils.
6. Body flattened with distinct oral and aboral surfaces.
7. There are no spacious prolongations of the coelom into the arms.
8. Anus is lacking.
9. Mouth and madreporite are situated on the oral surface of the body.
10. Gonads are pentamerous and the genital bursae usually act as the gonoducts.
11. Larva is Ophiopluteus.
It includes 3 orders –
(i) Order Oegophiurida:
Features:
1. It consists most of the fossil species except single living species.
2. No dorsal or ventral shields or bursae.
3. Madreporites at edge of disc.
Example:
Ophiocanops.
(ii) Order Phrynophiurida:
1. Dorsal arm shields are absent.
2. Arms are usually branched and can move vestically.
3. They can coil themselves around any object.
4. Spines are directed downward.
5. One madreporite at each inter-radius and the number corresponds with that of the stone canal.
6. Spines are often modified into hooks.
Examples:
Ophiomyxa, Asteronyx, Gorgonocephalus, Astrophyton, Astrogymnotes, Euryale, Astrothorax (Fig. 21.30D).
(iii) Order Ophiurida:
Features:
1. Mostly small ophiuroids, usually with 5 arms.
2. Arms are simple and un-branched.
3. Arms can move along the transverse plane of the body.
4. Dorsal arm shields are present.
5. Madreporite single.
6. Spines are directed outward.
The order includes most of the brittle stars.
Examples:
Amphiura, Ophiopholis, Ophiothrix, Ophioderma, Ophiocoma, Ophiolepis, Ophiostigma, Ophiactis, Ophiura, Ophiacantha, Sigsbeia.
3. Class Concentricycloidea:
They are called sea daisies and are known by single genus and two species that were discovered in 1986 from a submerged wood.
Features:
1. Minute (maximum 1 cm diameter), deep-water medusa-like bodies.
2. Two concentric water rings on the outer edge of the disc.
3. Coelom spacious.
4. Ambulacral system is absent.
5. No larval stage.
Example:
Xyloplax.
D. Subphylum Echinozoa:
Features:
1. Globoid or discoid echinoderms without radiating arms or brachioles (small arm-like processes).
2. Mouth and anus lie at opposite poles in earliest members.
3. Mostly unattached.
4. Hydrocoel forms a ring about the mouth.
The subphylum comprises 2 classes.
4. Class Echinoidea (Ordovician—Recent):
[Gk. echinos = a hedge-hog; eidos = form] Approx. 900 species.
The sea urchins, heart urchins, cake urchins are included under this class.
Features:
1. Body may be globular, heart-shaped, oval or disc-shaped.
2. Body orally and aborally flattend and without arms.
3. Body enclosed by a skeleton in the form of a continuous test, shell (= corona) of closely set of calcareous plates.
4. Spines movable.
5. Ambulacral grooves absent but surface is divided into alternate ambulacral and inter-ambulacral areas.
6. Ambulacral plates have pores for the passage of tube-feet.
7. Tube-feet highly extesible, provided with suckers and locomotory in function.
8. Mouth and anus surrounded by membranous peristome and periproct respectively.
9. Larva is Echinopluteus.
Echinoids may be divided into regular or irregular urchins.
The regular urchins are characterised by:
1. The test or corona is globular and shows pentamerous symmetry.
2. Spines are long and unusually thick and cylindrical (e.g., Eucidaris, Echinus, Strongylocentrotus, etc.).
3. Each inter-ambulacrum has two rows of plates.
4. The lantern of Aristotle is well developed.
5. Centric anus.
All sea urchins are included to this group and also sometimes classified as regularia (regular urchins) or endocyclica (centric anus), as a subclass.
Examples:
Sea urchins (Cidaris, Diadema, Arbacia, Salmacis, Echinus, Echinometra, etc.).
The irregular urchins or irregularia (e.g., heart urchins, sand dollars, and sea biscuits) are characterised by:
1. The test or corona is mostly flattened and the shape is either oval or round and they exhibit varying degrees of bilateral symmetry.
2. The spines are relatively shorter.
3. The ambulacral areas on the aboral surface form a five-pointed petaloid condition like the petals of a flower.
4. The tube-feet are mostly non-locomotory.
5. Anus eccentric (exocyclic).
The irregular echinoids are sometimes classified as irrgularia or exocyclica (anus ecentric) as a subclass.
Examples:
Sea biscuits (Clypeaster); Sand dollar (Encope, Mellita, Dendraster, etc.); Heart urchins (Echinocardium, Lovenia, Spatangus, etc.)
The class Echinoidea is divided into two subclasses:
Subclass A. Perischoechinoidea:
Features:
1. They are largely primitive fossil sea urchins of the palaeozoic seas. The earlist representatives are found in the Ordovician period. Bothriocidaris has been reported from Russia. The test is rigid and round in shape. Each ambulacrum has two rows of plates and interambulacrum has single row of plates. The typical lantern of Aristotle is absent. The madreporite is radially disposed.
(i) Order Cidaroida:
The order includes both extinct and existing echinoids.
Features:
1. Test round.
2. There are two rows of plates for each ambulacrum and interambulacrum.
3. Each interambulacral plate bears one large spine which is surrounded by small spines at the basal end.
4. Gills, sphaeridia are absent.
Examples:
Cidaris, Eucidaris (Fig. 21.31A), Phyllacanthus, Stylocidaris, Goniocidaris.
Subclass B. Euechinoidea:
This subclass contains the most living species of echinoids.
Superorder Diadematacea:
1. Sea urchins with perforated tubercles.
2. Spines are extremely long and pointed.
3. Gills usually present.
(i) Order Pedinoida:
Rigid test with solid spines
Example:
Caenopedina.
(ii) Order Diadematoida:
Rigid or flexible test with hollow spines
Example:
Diadema (Fig. 21.27F).
(iii) Order Echinothuroida:
1. Deep sea species with a delicate, flexible test.
2. Gills inconspicuous or lost.
Example:
Asthenosoma.
Superorder Echinacea:
1. Rigid test with solid spines.
2. Gills present.
(iv) Order Arbacioida:
1. Test globular.
2. Spines solid
3. Periproct with four or five plates.
Example:
Arbacia (Fig. 21.27C).
(v) Order Temnopleuroida:
1. Test sculptured in some species.
2. Camarodont lantern (Epiphysis of the lantern are greatly enlarged and meet the pyramids to form bar).
Examples:
Temnopleurus, Salmacis, Toxopneustes, Lytechinus, Tripneustes.
(vi) Order Echinoida:
1. Test non-sculptured and rarely oval with imperforate tubercles.
2. Camarodont lantern.
Examples:
Echinus, Paracentrotus, Echinometra, Echinostrephus, Strongylocentrotus.
Superorder Gnathostomata:
1. Lantern present.
2. Ambulacral plates single.
3. Mouth in the centre of oral surface but anus situated to the apical centre.
4. Irregular urchins.
(vii) Order Holectypoida:
1. This order includes extenct forms.
2. The test is regular.
3. Ambulacra are simple and do not show petaloid development.
4. Lantern of Aristotle present.
Examples:
Pygaster, Plesiechinus, Holectypus, Echinoneus, Micropetalon.
(viii) Order Clypeasteroida:
1. Members are called sand dollars.
2. Body irregular with flattened test.
3. Lantern and petaloids present.
4. Gills lacking.
5. Mouth centrally placed but anus excentrically placed.
6. Ambulacra at the aboral side are petaloid.
Examples:
Clypeaster (Fig. 21.31B), Arachnoides, Echinocyamus, Fibularia, Mellita, Encope (Fig. 21.31C), Rotula, Laganum, Dendraster (Fig. 21.31D).
Superorder Atelostomata:
Irregular urchins. Lantern absent.
(i) Order Spatangoida:
1. The members of this order are called heart urchins.
2. Body irregular with an oval test.
3. Lantern and the gills absent.
4. Mouth and anus excentrically placed.
5. Four of the five ambulacral areas have become petaloid at their aboral ends.
Examples:
Spatangus, Echinocardium, Plexechinus, Urechinus, Moira, Meoma, Lovenia, Brissopsis, Paraster, Hemiaster, Aeropsis, Palaeostoma.
(ix) Order Cassiduloida:
1. This order includes mostly the fossil forms.
2. Test round and oval in outline.
3. Lantern of Aristotle absent in living forms.
4. Ambulacra become petaloid at the aboral end.
5. Genital plates usually fused with, the madreporite.
Examples:
Echinolampus, Apatopygus, Cassidulus, Anochamus, Tropholampas.
5. Class Holothuroidea (Devonian—Recent):
[Gk. holothurion = a water polyp; eidos = form]; Approx. 1200 species.
Features:
1. The members of the class are known as sea cucumbers.
2. Body cylindrical and exhibits bilateral symmetry.
3. Mouth and anus located at the opposite extremities of the body.
4. Skin soft and thin and without spines and pedicellariae.
5. Oral podia modified as tentacles.
6. Skeleton reduced to microscopic ossicles.
7. Surface of the body may exhibit five ambulacral areas.
8. Stone canal without the external opening.
9. Tube-feet locomotory in function and restricted to the five ambulacral areas.
10. Larva is auricularia.
(i) Order Dactylochirotida:
1. Primitive sea cucumbers.
2. Tentacles simple.
3. Body U-shaped and enclosed within a flexible test.
Example:
Echinocucumis.
(ii) Order Dendrochirotida:
1. Oral tentacles are arborescent (tree like in appearance) and dendritic, i.e., with tree-like branches and number is usually 10 (upto 30). Tentacles are not provided with ampullae.
2. Tube-feet present and may spread all over the body or may be restricted to the ambulacral areas.
3. Respiratory trees present.
4. Respiratory trees lack Cuvierian tubules.
5. Usually shallow water forms.
Examples:
Cucumaria (Fig. 21.29E), Thyone, Trachythyone, Ypsilothuria, Psolus, Pentacta, Sphaerothuria, Leptopentacta.
(iii) Order Aspidochirotida:
1. Tube-feet numerous.
2. Oral tentacles shield-like or peltate, i.e., branched from central stalk and the number is usually 20 in most cases but varies 15 to 30 in some.
3. Pharyngeal retractor muscles absent.
4. Respiratory trees with Cuvierian tubules well-formed.
5. Usually shallow water forms.
Examples:
Holothuria, Actinopyga, Stichopus, Bathyplotes, Synallactes.
(iv) Order Elasipodida:
1. Tube-feet few.
2. Oral tentacles shield-shaped or peltate and their number varies from 10-20.
3. Body generally flat ventrally and mouth usually ventral.
4. Pharyngeal retractor muscles absent.
5. Tentacular ampullae absent.
6. Respiratory trees lacking.
7. Most of the deep sea species.
Examples:
Deima, Oneirophanta, Laetmogone, Benthodytes, Peniagone, Pelagothuria (Fig. 21.28B), Elpidia (Fig. 21.32B).
(v) Order Molpadida:
1. Regular tube-feet absent.
2. Oral tentacles small, un-branched, digitate and the number varies from 10-15 (usually 15 but rarely 10).
3. Tentacular ampullae present.
4. Respiratory trees present.
5. Posterior end of the body narrowed to a tail.
Examples:
Molpadia (Fig. 21.32A), Paracaudina, Acaudina, Caudina.
(vi) Order Apodida or Synaptida:
1. Body elongated and vermiform.
2. Tube-feet absent.
3. Tentacles digitate or pinnate and the number varies from 10-25.
4. Tentacular ampullae vestigial or absent.
5. Water vascular system greatly reduced and the radial ambulacral vessels absent.
6. Respiratory trees absent.
Examples:
Synapta, Synaptula,
Opheodesoma (Fig. 21.29B), Leptosynapta, Protankyra, Polycheira.
4. Phylogenetic Considerations of Echinoderms:
The phylum Echinodermata exhibits variety of forms. They differ from one another by specialised features. Despite apparent diversities, they possess striking similarities in development and in their basic structural organisation.
They have many common features:
1. The eggs are small and yolked.
2. The cleavages are holoblastic and radial.
3. Gastrulation involves invagination.
4. The blastopore has posterior location.
5. The coelom is developed as the outpockets from the archenteron (enterocoelic in origin).
6. The larvae are free-swimming and have bilaterally symmetrical bodies.
7. In all adult Echinoderms, adjacent to the epidermis there exists calcareous ossicles constituting the skeleton of the body. The disposition of the plates varies in different forms.
These common features suggest the idea that all Echinoderms have emerged out of a common ancestral stock where such structures were present. It is also regarded that the ancestral forms with bilateral symmetry, are the forerunners of the group.
Although fossils were quite abundant since Precambrian time, no tangible explanation can be obtained regarding the ancestry of Echinoderms from the palaeontological angle.
The presence of superficial radial symmetry in adult Echinoderms leads to many confusing issues. Because of radial symmetry, some zoologists have tried to link them with the Coelenterates.
But the presence of bilateral larval forms and underlying bilateral symmetry in some adult Echinoderms suggest that they have arisen from bilaterally symmetrical ancestor and the radial symmetry in adult is a secondary acquisition (Fig. 21.33).
With all probabilities, some unknown Precambrian forms with bilateral symmetry are to be regarded as the ancestor of Echinoderms. Eocrinoids with stalk or stalk-less body, five ambulacra at the oral end and pentamerous symmetry give a superficial resemblance to crinoids and forms a sort of ancestor of pentaradiate adult echinoderms.
Two exactly opposite views exist on this particular issue. According to the first concept the hypothetical ancestor is the Dipleurula larva but other school holds that the Pentactula larva is the ancestor.
Dipleurula concept:
The view that the Dipleurula larva was the hypothetical ancestral form of the Echinoderms was propounded by Bather (1900).
This larval form has following features:
1. Bilaterally symmetrical body with pre- oral lobe.
2. Absence of skeletal formations.
3. Ventrally placed mouth and anus.
4. Presence of three paired sacs (axocoel, hydrocoel and somatocoel) with two water-pores.
It has been imagined by many workers that this larva holds the key of Echinoderm ancestry. But it was not possible to explain the derivation of some vital Echinoderm features, viz., the water vascular system and its development from the coelom.
According to Bather, water vascular system originates as three ciliated grooves which become five by the subsequent subdivision of the two lateral grooves. The available interpretations on the origin of the water vascular system are not supported by embryological data. Embryological studies showed that the water vascular system arose as the outgrowths of the body wall in the form of tentacles.
Pentactula concept:
The pentactula concept of the Echinoderm ancestry has certain advantages over the dipleurula stage. This idea was first conceived by Semon (1888) and later developed by Burry (1895), Hyman (1955) and many others. According to them the Pentactula larva occupies the next evolutionary rank over the dipleurula larva.
The pentactula larva has five tentacles around the mouth and the hydrocoel becomes separated from the rest of the coelom to form the water vascular system. The recent inclination is to hold the view that the Echinoderms have descended from a free-swimming common ancestor, possibly the pentactula larva, and from the pentactula stage onwards the divergence has actually started.
As regards the inter-relationships between the different groups of the phylum Echinodermata, it was suggested by early workers that the subphylum Eleutherozoa consisting of the sea-stars, sea-urchins, sea- cucumbers and brittle-stars has arisen from the subphylum Pelmatozoa which included the crinoids and others.
Early workers also suggested that the sea-stars and sea-cucumbers had many common features specially at the larval stages. Both of them were held to be related to crinoids more closely and they had possibly diverged very early from the Pelmatozoan ancestor.
On the other hand, ophiuroids and echinoids were regarded to be closely related with each other due to presence of striking similarities in between their larval forms and also due to the presence of similar sterols (Bergmann, 1949) and creatine phosphate (Yudkin, 1950; Florkin, 1952) in both ophiuroids and echinoids.
Such similarities led early workers to establish a relationship between ophiuroids and echinoids and both were regarded to have diverged subsequently from the Pelmatozoan ancestor.
Eminent palaeontologists like, Bather, Marcus, Mortesen held that all the classes of the subphylum Eleutherozoa arose from Edrioasteroidea, an extinct group of Pelmatozoans, because of similar disposition of ambulacral pores, flexible theca, stalkless body and many other features. Shrock and Twenhofel suggested that only the holothurians and echinoids have evolved from Edrioasteroidea.
But recent workers, like Fell (1965) Nichols, 1969 and others, have totally discarded the views of all early workers and have reported two conflicting views.
On one-hand, some workers claimed that Ophiuroids and echinoids are closely related due to striking similarities between ophiopluteus and echinopluteus larvae and that asteroids and holothurians are related due to the larval resemblance (see Fig. 21.33) are shown to be in total disagreement with the results obtained from morphological and palaeontological studies.
Nichols (1969) also reported that biochemical studies of pigments indicate ophiuroid—echinoid and asteroid— holothuroid relationship.
Recent researches show that the larval relationship between ophiuroids and echinoids in one hand and between asteroids and holothuroids and that of the hemichordates on the other are due to convergent larval evolution.
Modern works advocate that the apparent dissimilarities between ophiuroids and asteroids are due to divergent larval evolution. Fell showed that the echinoderms show convergent patterns of development among unrelated groups (Asterozoa, Echinozoa and Crinozoa) and divergent patterns of development among related groups (Ophiuroidea).
It is also indicated that both ophiuroids and asteroids are closely related to each other and they have certainly descended from common ancestors akin to the fossil Eocrinoida. The eocrinoids and crinoids again had a common ancestry. On the other hand Holothuroidea and Echinoidea had a common ancestry.
Thus modern researches suggest that the larval affinities cannot act as a guide in establishing the phylogenetic relationships between different classes of surviving echinoderms. Moreover, the superficial biochemical similarities between ophiuroids and echinoids may also be a result of physiological convergence for similar mode of living.
The origin of and inter-relationship between different classes of echinoderms are shown in Fig. 21.34.
On the basis of morphology and palaeontology early ophiuroids can establish the relationship with asteroids rather than echinoids by comparing the skeletal structure. Again early asteroids can show some striking resemblances to crinoids rather than holothuroids on the basis of arm structure. So palaeontological and morphological evidence help to construct a classification based on adult animals rather than larval forms.
According to Nichols (1969), crinoidea includes the oldest known members and therefore may be considered as the most primitive echinoderms. Asteroids and ophiuroids to be closely related groups on the basis of skeletal structure. Ophiuroids and echinoids may be related on the basis of lacking of open ambulacral groove.
5. Research on Indian Echinoderms:
Prof. F. J. Bell (1887-1902) of the King’s College, London who was the first to report on the echinoderms of India along with the Edgar Thurston (1887-1894) of the Madras Museum. Bell (1887) reported 17 holothurian species from the Andaman and Nicobar Islands. Koehler and Vaney (1908) reported 51 holothurian species from Andaman and Nicobar Islands.
Gravely (1941) listed 21 echinoderm species from the Madras (Chennai) beach. Kurian (1953) reported 4 species from Travancore coast. Chacko et al. (1956) published a list collected from Krusadai Island in the Gulf of Mannar. Grideon et al. (1957) mentioned a few echinoderms only up to genus level from the Gulf of Kutch. Sanne and Chhapgar (1962) listed 15 species from intertidal region of Bombay (Mumbai). Gopalkrishnan (1969) reported species of holothurians from the Gulf of Kutch.
In the midsixties some work on the reproductive physiology was initiated by Prof. S. Krishnaswamy, Krishnaswamy and Krishnan (1967), K. S. Rao (1965, 68) and Rahman (1966, 1968). Dr. S. Jones initiated work on the associations in general and echinoderms in particular. Jones listed over 100 species known from the Gulf of Mannar and Palk Bay.
Nagbhusnam and Rao (1969) listed 6 species from Orissa coast. James (1983) reported nearly 200 species of echinoderms from various places along the east and west coasts of India, the Andaman and Nicobar Islands and the various islands of Lakshadweep.
Soota, Mukhopadhyay and Samanta (1983) listed 18 unnamed holothurian species of the Andaman and Nicobar Islands which were collected by them and some were deposited in the Zoological Survey of India.
Badal Chandra Bharati Goswami (1992) recorded 5 speices from the Digha coast. In India, about 330 species have been recorded from the different coasts. Within this only 50 species have been recorded from the entire west coast. A. H. Clark (1912) reported about 75 crinoid species from the Indian region in his list of Indo-West Pacific species.
6. Habit and Habitat of Echinoderms:
Echinoderms are exclusively marine animals. They inhabit all the seas and in all latitudes. They are usually absent in colder areas, excepting Crinoids which are not uncommon in arctic and antarctic regions. Echinoderms are found from the intertidal zone to the depth of about 6,000 m.
Almost all the Echinoderms are benthonic and live in all types of sea bottoms. Asteroids crawl on the bottom and a few forms, belonging to the family Benthopectinidae, swim by the arms. The Holothurians remain adhered to the rocky bottoms and conceal themselves under rocks.
Some Holothurians like Holothuria, Stichopus, Actinopyga, are adapted to the sandy bottom. Some of them remain partly or wholly buried in sandy or muddy bottoms. Leptosynapta spends entire life being completely buried in soft bottoms and they move under the surface. Echinoids are also benthonic animals and keep the oral surface of the body in contact with the substratum.
Some Echinoids are rock-borers and make bores like honey-combs in the rocky wall. The typical rock-boring Echinoids are Psammechinus miliaris and Paracentrotus livides. The Ophiuroids inhabit all sorts of sea-beds. They usually hide under rocks and seaweeds. They remain attached to other objects by their flexible arms.
They exhibit various types of movement by the arms. Some Ophiuroids have the habit of burrowing in sand as seen in Amphiura chiajei. The crinoids are shallow-water inhabitants, except the stalked forms which occur mostly in deep sea ranging from 200-5000 m.
Most of the Echinoderms are sluggish animals and move very slowly. They can either crawl on the surface or may swim in water by the arms. The ophiuroids are the most active forms amongst the Echinoderms. The Crinoids are more or less sedentaric animals.
Most of the adult Crinoids are stalked and remain fastened to the bottom by aboral side of the body directly or through a stalk. The Jeep sea forms are generally stalked. A few Crinoids detach themselves from the stalk in adults and usually lead free and pelagic life.
Echinoderms are gregarious animals and usually live in large groups. They are mostly nocturnal. They are bottom feeders and eat all sorts of food available in the sea bottoms. Most of them are carnivorous but several herbivorous forms are also known.
7. Size of Echinoderms:
The sizes of Echinoderms are relatively moderate. The largest Asteroid known is Pycnopodia helianthoides which measures about 90 cm. The largest urchin living in the deep sea realms is Echinosoma hoplacantha whose shell is about 31 cm across.
In another urchin, Diadema, the spines are about 30 cm long. Synapta maculata, a Holothurian, has 16 cm long body with the diameter of about 6 cm. The stem of some fossilised Crinoids is about 2 m in length. As regards the smallest representatives of the group, it is seen that some fossil sand dollars measure only 2.5 cm.
8. Shape and Symmetry of Echinoderms:
The phylum Echinodermata constitutes a very well-defined group. They exhibit distinct radial symmetry in adults, excepting some Holothurians where the radial symmetry is not marked externally. The disposition of both internal and external structures also exhibits radial symmetry in Echinoderms which is an exception among the Coelomata.
The radial symmetry in adult organisation is acquired at the time of metamorphosis because the larval stages of all Echinoderms have bilaterally symmetrical bodies. All the Echinoderms have a pentamerous body plan and have distinct oral and aboral surfaces. The terms ‘dorsal’ and ‘ventral’ are not applied in Echinoderm organisation.
The mouth is centrally situated at the oral surface excepting a few Echinoids, Holothurians and Actinometra (Crinoid) where the position of the mouth is slightly shifted from its original place. But the position of anus varies quite greatly (Fig. 21.35) and it is located excentrically. In some Echinoids and Crinoids, the anus is placed on the oral surface. The anus may be totally wanting in a few adult Asteroids and Ophiuroids.
The symmetry of the body has moulded all the organs of the body. Externally, the radial symmetry is exhibited by radii and inter-radii. The radii are marked by rows of tube-feet and the inter-radii are the portions of the body between the radii. But in some Holothurians, the tube-feet may spread over the inter-radii of the body.
The tube-feet are usually restricted to the oral surface (excepting Holothurians and Echinoids where the tube-feet extend up to the aboral side to some extent) which is also called actinal or ambulacral surface. The other side (without tube-feet) is called abactinal or adambulacral surface.
The body is prolonged into arms in the direction of the radii in Asteroidea, Ophiuroidea and Crinoidea but in roundish Echinoids and sausage-shaped Holothurians, the aboral surfaces are inconspicuous due to the extension of the ambulacral surface.
In all the members of the subphyla Asterozoa amd Echinozoa, excepting the Holothurians, the mouth is directed downwards. In Holothurians, the mouth is horizontally directed and they apply one side of the body to the ground. But in Crinoids, the mouth is directed upwards.
Presence of tube-feet or podia is the characteristic feature of the phylum. In Asteroidea, Echinoidea and Holothuroidea, the tube-feet are provided with terminal suckers and they help in locomotion. In other forms, they are variously modificed to sub-serve other functions, like respiration, food-collection and tactile sensation.
9. Body Wall and Skeleton of Echinoderms:
The epidermis is usually ciliated except in Holothuroids and Ophiuroids. The epidermis usually contains gland cells and sensory cells. The characteristic dermal ossicles vary quite greatly. They may remain scattered or may be firmly united so as to form an armour. Despite great diversities in form, they are possibly homologous in all Echinoderms.
The ossicles are formed as the deposition of calcareous substances in the connective tissue matrix. The dermal plates are spiny in most cases and the spines are mainly protective in function. They may also help in locomotion in some cases. In paxillosids and valvatid sea stars, the raised part of ossicles of the aboral surface is crowned with movable short spines, called paxilla help in burrowing.
The spines and other processes derived from them are movably placed. In Asteroids and Echinoids, some of the spines become specialised into pedicellariae. The detailed structure of the pedicellariae has been described in the example part of the phylum. These are nothing but two or three spines arranged as pincers.
The pedicellariae are primarily protective in function. The dermal plates are quite distinct in some Echinoderms; in others they are not clearly understandable. However, the plates are broadly distinguishable into oral and aboral plates. The oral plates are five in number and are inter-radially placed. They can be easily distinguished in the Crinoids and Ophiuroids.
The aboral plates are quite distinguishable in early developmental stages (Fig. 21.36). The aboral plates, in typical cases, consist of a central plate which is surrounded by five radially disposed infrabasals and five interradially placed basals.
Beyond the circle, five radially arranged radials are placed. Both the oral and aboral plates are absent in Holothurians. The infrabasals as well as the radials are lacking in Echinoids. Other details regarding the skeletal system are dealt under the different examples of the phylum.
10. Digestive System of Echinoderms:
The alimentary canal in Echinoderms shows great variation in different groups. The alimentary canal becomes coiled in most forms. In Ophiuroidea and Asteroidea the alimentary canal is axial. The position of the mouth and anus varies greatly in different classes. The anus is usually excentrically placed excepting Holothurians.
In Crinoids, the mouth and anus are situated on the same side of the body. The anus is lacking in Ophiuroids and in a few adult Asteroids. The alimentary canal bears various diverticula, such as siphon in Echinoids, respiratory trees in Holothurians, cardiac and pyloric caeca in most Asteroids and intestinal diverticula in Crinoids. Lack of separate glandular appendages is the most remarkable feature in Echinoderm anatomy.
11. Coelom of Echinoderms:
The coelom in the adult Echinoderms is represented as several distinct spaces. It develops from a diverticulum of the embryonic enteron. The diverticulum subsequently becomes separated from the enteron and forms a number of sacs—the hydrocoel and the splanchnocoel.
The water vascular system arises from the hydrocoel, and the splanchnocoel transforms into several systems of spaces:
(1) The body cavity proper or the perivisceral cavity develops from the right and left posterior coelom in the larva. It has no connection with the exterior except in Crinoids where the water-pores open into it.
(2) The axial sinus varies greatly in development. It usually develops from the anterior larval coelom. The stone canal opens into it and communicates with the exterior through water-pores or with the coelom as in most Holothurians.
(3) The sinus system or the perihaemal spaces. This part develops as out-pushing of the posterior and anterior larval coeloms. This part of the coelom forms a covering of the haemal channels. It consists of a circumoral space and five radially placed tubes. This system is not present in Crinoids. The aboral circular sinus is also included in this system.
The coelomic spaces have an epithelial lining which is ciliated in most places. It contains albuminous fluid with floating amoebocytes which are phagocytic in function. Several types of amoebocytes of different sizes and appearances have been distinguished.
Another type of corpuscle with vibratile tail is also recorded in the coelomic fluid. They are designated as vibratile corpuscles. They set forth a constant circulatory movement of the coelomic fluid. In Holothurians, a good number of flattened nucleated cells containing haemoglobin are present.
12. Haemal System or Blood Lacunar System of Echinoderms:
The existence of blood vascular system in Echinoderm cannot be definitely stated. The haemal system in echinoderm plays the role of blood vessels. But the fluid contained in this system does not show any circulatory movement. This system comprises a peculiar connective tissue of doubtful nature.
The haemal system is present in all the Echinoderms and is welldeveloped in Asteroidea and Ophiuroidea. The haemal system is greatly reduced in Crinoidea. The haemal system is almost same in all the classes of Echinoderm excepting a few minor modifications regarding positional changes of the madreporite.
In Echinoderms, the haemal system consists of an oral ring with five radial canals which terminate blindly at the aboral end. The oral ring gives off a gastro-intestinal system which becomes quite complicated in most of the cases. Conspicuous development of the gastro-intestinal system suggests that its primary function is nourishment.
13. Excretory System of Echinoderms:
The process of excretion in Echinoderms in not fully known. There is no specialised organ in Echinoderm which can be assigned to be excretory in function. With all probabilities, some organs associated with the water vascular system or with the axial sinus are responsible for the elimination of the nitrogenous waste products.
14. Nervous System of Echinoderms:
The nervous system is modified in different classes of Echinoderm.
This system consists of:
(1) Ventral nervous system;
(2) Deep oral nervous system and
(3) Apical nervous system.
The central nervous system is well-developed in different classes of Echinoderms. This system is placed superficially and consists of diffused sub-epithelial nerve plexus which becomes concentrated in the different parts of the body. It is sensory in nature and supplies nerves to the integument, tube-feet and gut.
The ectodermal part of the plexus is called ectoneural and the endodermal part is designated as endoneural. The ectoneural plexus becomes concentrated around the mouth as the circumoral nerve ring which in turn gives radial nerves along the radii. In Echinoidea, Ophiuroidea and Holothuroidea, the circumoral nerve ring and radial nerves are placed in the wall of the epineural canal.
The ectoneural plexus prolongs into the tube-feet, spines and pedicellariae. The endoneural plexus is well formed in Asteroidea and forms perioesophageal nerve ring around the mouth opening. The deep oral nervous system consists of two nerve cords (Lange’s cords) in each radius. These two nerve cords lie in the ectoneural system and are separated from the radial nerve by connective tissue layer.
They are mesodermal derivatives and motor in nature. The apical nervous system consists of a cord situated in the mid-dorsal line of the body. It develops from the dorsal peritoneum. This system is best developed in Crinoidea and is altogether absent in Holothuroidea. It is exclusively motor in nature and mesodermal in origin.
The sense organs are mainly the tactile organs. The tube-feet are exclusively sensory in function in majority of the Echinoderms. The terminal tentacles are sensory units in starfishes. They are tactile organs situated one at the tip of each arm. This spines are also highly sensitive.
The pigmented spots situated at the tip of the arms of Asteroidea are probably photosensitive. The shining spots on the skin of an Echinoid, Diadema, are also assigned to be visual in function. The otocysts of Holothuroidea and Sphaeridea (modified spines) of Echinoidea are the organs of special sense. Besides these structures, numerous neurosensory cells are scattered all over the body which are either tactile organs or chemoreceptors.
15. Reproductive System of Echinoderms:
Sexual reproductions:
In Echinoderms, the sexes are separate except a few hermaphroditic forms, such as Asterina gibbosa, Cucumaria frondosa and Amphiura squamata. The reproductive organs in Echinoderms are quite peculiar in nature. MacBride has shown that the reproductive organs are composed of cells derived from coelomic epithelium.
The reproductive organs are connected with a cellular cord, called genital rachis. Existence of the genital rachis in Holothuroidea is questionable. A cord having germ cells passing along the genital duct towards the body wall is comparable to the genital rachis of others.
The reproductive organs consist of tufts of branched, paired and inter-radially disposed bodies. They open directly on the surface. In Crinoidea, the reproductive organs are contained in pinnules which are devoid of an opening to the exterior. The mode of discharge of genital products in this case is not known.
In Asteroidea, Ophiuroidea and Echinoidea, the gonads are oriented pentamerously in the inter-radii. In Asteroidea, there are five pairs of gonads which open usually on the aboral surface. In a few genera under the class Asteroidea, the gonads are numerous in number.
In Holothuroidea there is only one branched gonad which may be imperfectly divided into two. But the single duct from the gonad opens to the exterior on the surface near the mouth. The reproductive cells, when ripe, are discharged directly into the sea.
Asexual reproduction:
Asexual reproductive by fission is observed in many Echinoderms, especially in Asteroidea; Ophiuroidea and Holothuroidea, In Asteroidea and Ophiuroidea, a line of fission passes through the central disc, while in Holothuroidea it passes transversely along the axis of the body.
Spontaneous reproduction by fission is reported in Asteriid genera (Coscinasterias, Sclerasterias, Stephanasterias, Linckia and Allostichaster). Crozier (1920) found that in the first animal asexual reproduction is confined to summer months and normal gametic reproduction occurs in winter months.
16. Regeneration in Echinoderms:
The echinoderms are endowed with remarkable power of regeneration of their lost parts by autotomy (self-mutilation). The power of regeneration is relatively poor in Echinoidea, but in Asteroidea and Ophiuroidea, it is quite extensive.
Regeneration of whole body from a single arm is recorded in starfishes. If arms or a portion of the central disc is extirpated or lost mechanically they can regenerate the lost portions. In Crinoidea and Holothuroidea the power of regeneration is so profound that the internal organs or portions of them are capable of being regenerated.
17. Development of Echinoderms:
In all Echinoderms, the eggs are fertilized in sea water. Almost all the echinoderms pass through characteristic free-swimming and bilaterally symmetrical larval stages (Fig. 21.39).
In Asterina gibbosa, free-swimming larva is absent as the development occurs in the brood of the mother. In a few holothurians, development occurs in the coelom and in the genital tubes in Chiridota contorta. Development in Stichaster nutrix takes place in the stomach of the mother.
The Echinoderms, where free-swimming larval forms are present, have small eggs. But in forms where development occurs in brood or body cavity or in stomach, the eggs are relatively large in size owing to greater quantity of yolk. The fertilized eggs undergo total cleavage (holoblastic) and a large number of cells (blastomeres) are produced. These cells arrange themselves so as to form a hollow one-layered blastula.
The blastula by invagination at its one pole transforms into a gastrula. The invaginating cells form the endoderm and the outer layer becomes the ectoderm. The archenteron communicates posteriorly with the exterior through the blastopore. Mesenchymal networks in the form of nucleated protoplasmic processes are formed between the ectoderm and endoderm.
These networks give rise to muscles, connective tissues and calcareous ossicles. The archenteron acquires a new opening to the exterior at the anteroventral side, called larval mouth. As development progresses, the anus is shifted ventrally and the cilia present all over the body become localised into definite bands. The disposition of the ciliated bands differs in different larval forms.
Fate of larval mouth and anus:
The fate of the larval mouth and anus varies quite greatly among the different classes of the Echinodermata. In Asteroidea and Echinoidea, both of these closes and the adult mouth and anus are formed anew. In Holothuroidea, both of these persist in adults. In Ophiuroidea, anus is lacking in adults but the larval mouth persists. In Crinoidea, both larval mouth and anus are absent.
18. Affinities of Echinoderms:
The echinoderms, especially their larval forms, attract the attention of many Zoologists due to the presence of many striking similarities between themselves and between different other groups of animals.
Relationship with Annelida:
A number of early workers have established affinities between the trochophore larva of annelids and some echinoderm larvae on the basis of the presence of similar ciliated bands and some other superficial similarities.
But these affinities are not based on any scientific ground because cleavage pattern is spiral in annelida but radial in echinoderms; coelom formation is schizocoelic in annelids but enterocoelous in echinoderms.
Relationship with Brachiopoda:
Some superficial similarities are also noted in between the early developmental stages of Brachiopoda and Echinodermata.
The similarities are:
1. Cleavage is holoblastic,
2. Blastula is a coeloblastula,
3. The coelom is enterocoelous in the members of the class Articulata of phylum Brachiopoda,
4. The members of class Articulata have free-swimming larval stage. However, these affinities are only superficial.
Relationship with Chordata:
The most convincing affinities are noted between the echinoderms and the chordates. Hence many workers regarded the echinoderms to be the nearest group to the chordates. However, modern workers do not support the contention and they hold that the echinoderms and the chordates diverged separately from a common basic ancestor.
The affinities are discussed below:
1. Mesodermal skeletal substance is present in both.
2. Presence of infra-epidermal nervous system in hemichordata.
3. The perforations on the calyx of carpoid echinoderms are compared with pharyngeal gill-slits of Amphioxus.
4. Needham (1932) has tried to show a relationship between these two groups by analysing biochemical evidences. Invertebrates have the phosphogen in the form of arginine phosphate whereas chordates usually have creatine phosphate. But the echinoids among echinodermata and hemichor-dates among Chordata have both arginine phosphate and creatine phosphate.
5. Wilhelmi (1942) has shown similarities between the two groups by serological tests as well.
6. Cleavage is radial, holoblastic.
7. Blastopore changes into anus.
8. Enterocoelous mode of coelom formation.
9. The similarities between adult echinoderms and chordates are very few, but the affinities between the larval forms are highly notable.
Metschnikoff (1869) tried to show the following affinities between the torn aria larva of Balanoglossus and the bipinnaria and auricularia larvae of the echinoderms:
1. free-swimming and bilateral symmetrical larvae in both,
2. transparent body with similar ciliated bands,
3. enterocoelous coelom with similar disposition,
4. similar location of mouth and anus,
5. the madreporic vesicles in bipinnaria are thought to be homologous with heart vesicle of Balanoglossus.
Significance of echinoderm larvae:
Bather (1900) claimed common ancestry of hemichordates and echinoderms from the dipleurula larva. The genealogical tree given in 1957 by Anderson and Guthrie and the phylogenetic tree given in 1948 by L. H. Hyman in collaboration with Prof. YV. K. Fischer who also supports the same idea, (vide the phylogenetic trees (Figs. 21.40 and 21.41).
Muller and Bateson again claimed that the tornaria larva and dipleurula larva had evolved form a common ancestral source.
De Beer and Garstang hold that the tornaria larva, the dipleurula and pluteus larvae are living relics from a very remote period when the echinoderms and chordates were not diverged.
The neotenous theory propounded by Garstang in 1894, 1928 holds that the chordates arose from some neotenous form of auricularia larvae. The sequence is—Auricularia larva (Echinodermata) → Tornaria larva (Hemichordata) → Tadpole (Ascidia) → Neoteny → Free swimming Chordates.
The adoral ciliated band of auricularia presumably functions to convey food particles in the oral aperture, and for this it has been converted to the floor of the pharyngeal cavity. Garstang suggests that endostyle was derived from this loop of the adoral band of the auricularia larva.
An atrium was developed in course of time to protect the gills. The fishlike swimming larva of tunicates is held by him to be formed from the development of muscles along the sides of the elongated body.
The ciliary bands were then pushed upwards and subsequently rolled up with the underlying nerve plexus to form the neural tube. Fig. 21.42 shows Garstang’s idea of derivation of the protochordate from echinoderm larva.
Most of the modern workers like Berrill (1955), Bone (1979) have supported the main theory of Garstang but deny the origin of todpole. The tornaria larva of Hemichordate is much more similar to the larvae of echinoderms but differs in its mode of origin and its structure. Young (1981) supported the neotenous larval theory of Garstang.
Therefore, from the above discussion it may be concluded that the phylogenetic relationship between echinoderms, hemichordates and chordates is more convincing, and chordates may have evolved from non-chordates, probably the echinoderms.
But Fell (1963), Pawson and others deny the ancestry of chordates from any form of echinoderms arid they also deny the relationship between the tornaria larva and the echinoderm larvae. Many counter-arguments have been put forward by them.
1. Protocoel is unpaired in Balanoglossus, but paired in echinoderms.
2. Extant echinoderms lack pharyngeal gill-slits.
3. Bipinnaria larva lacks telotroch.
4. The tornaria larva has a characteristic apical plate with eye-spots.
Fell and many others hold that the affinities between tornaria and echinoderm larvae are not based on any phylogenetic relationship but are merely due to larval convergence for similar mode of life.
In Fell’s own words “It therefore follows that within the phylum (Echinodermata), larval similarities do not indicate taxonomic affinities. Extrapolating beyond the phylum, so as to deduce taxonomic affinities on the basis of resemblance between auricularia and tornaria is, therefore, inadmissible. The unreliability of larval structure in the echinoderms as a guide to phylogenetic affinity is further illustrated by the occurrence of divergent patterns of development within related groups (e.g., Asterozoa, Echinozoa, Crinozoa).”
The presence of similar biochemical substances may not necessarily speak of any phylogenetic relationship and may be due to convergence for similar modes of physiological activities. Moreover, creatine phosphate has been found to occur also in other animals phylogenetically remote from the chordates, such as in sponges (Robin, 1954/ and in many polychaetes (Hobson and Recs 1955).
The study of larval forms and adults of echinoderms reflects a case of retrogressive metamorphosis because the adult echinoderms possess many primitive features than the larval forms.
The features of adults are comparable with lower invertebrates (e.g., Cnidaria), such as radial symmetry, lacking of distinct head, absence of anterior and posterior sides, etc. and the advanced larvae of echinoderms metamorphose into primitive adults. Hence it is an example of retrogressive metamorphosis.
Views regarding the larval phylogeny:
MacBride (1914):
Dipleurula larva was of fixed type and gave rise to the free-swimming forms of Antedon or Yolk larva.
Hyman’s synthetic view (1955):
Dipleurula was remotely related non- echinoderm forms for their bilateral symmetry and went through a sessile stage of radial forms to reach the echinoderm status. This radial form Pentactula stage where reorganisation took place and gave rise to free- swimming and attached forms parallely (Fig. 21.43).
H. B. Fell (1963):
Supports free-swimming origin of echinoderm larvae and their phylogenetic correlation through Vitellaria.
Parker and Haswell (1972):
Dipleurula larva was free-swimming, gave rise to the free-swimming forms through the free-swimming Antedon on one hand and to the fixed type through the fixed Antedon forms, on the other.
Barrington has summarised the work of other workers like Berrill (1955), Bone (1960), Carter (1957), Marcus (1958) and Whitear (1957) and has proposed that the echinoderms, the pogonophores, the hemichordates and the rest of the chordates arose separately but directly from a common bilaterally symmetrical sessile or semi-sessile ancestor with tripartite body and coelom, ciliated larval stage and ciliary mode of feeding from external source (Fig. 21.44).