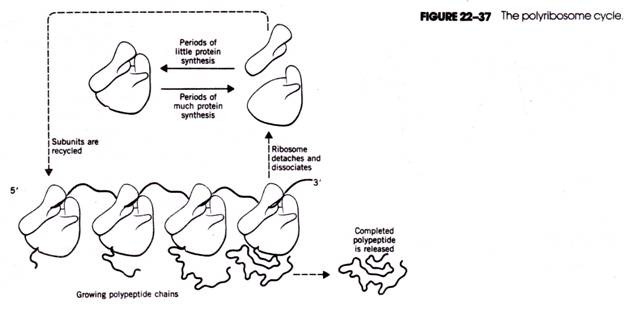
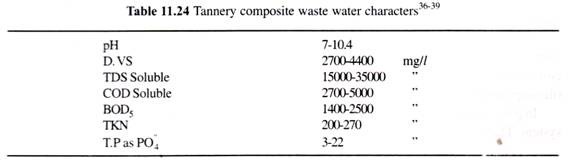
In this article we will discuss about:- 1. Distribution of Cycas 2. Morphological Features of Cycas 3. Internal Structure 4. Reproduction 5. Economic Importance.
Distribution of Cycas:
It is the only genus of the family Cycadaceae, which is represented in India. It has got about 15 species (Sporne, 1965), 20 species (Willis, 1966) which are widely distributed in Eastern as well as Western hemisphere from Madagascar, Eastern coast of Africa to Japan and Australia touching China and India.
In our country a few of the species are found growing abundantly in the South Andaman and Nicobar islands, Madras, Mysore, Malabar and in North East in Bengal, Assam, Nepal and Sikkim. A few of the species are also found in Burma and Ceylon.
The following species are found in India:
C. circinalis:
Plants are about 12 to 15 feet tall. Leaves are 5 to 9 feet long. It is distributed in western part of peninsular India, Malabar. Orissa hills, Andhra, Madras to Ceylon upto 3500 ft. The plants are also cultivated in Indian gardens. In Hindi it is called as Jangli-Madan mast-ka-phul.
C. pectinata:
Plants are about 8 to 10 feet tall. Leaves are 4 to 6 feet long. It is commonly found growing in Nepal. Sikkim, in Assam Khasia Hills, East Bengal and Burma. In Nepal it is commonly called as Thankal.
C. beddomei:
Plants are about 40 cm. tall. Leaves 3 feet long. It is naturally found growing on dry hills of Cuddapah in Andhra Pradesh, Madras, Malabar Java. Locally it is called as Per-ita.
C. rumphii:
Plants are more than 12 feet tall. Leaves 4 to 5 feet long. It is found growing in Andaman and Nicobar islands, Burma etc. It is also cultivated in Indian gardens. In Tamil it is called as Kama, Paiyindu.
C. revoluta:
Plants are upto 10 feet tall. Leaves 3 to 5 feet long. It is a native of China and Japan and locally called as Tesso. In our country it is mostly cultivated in gardens and locally called as Sago palm. Due to its primitive characters it is also known as living fossil. Exceptionally it has reached a height of about 20 feet in gardens of Taj Mahal Agra (India).
C. siamensis:
Trunk geophilous, tuberous but at times 6 feet tall.Leaves upto 4 feet long. It is a native of Burma, Cochin, China etc. Locally in Burma it is called as Mondaing. Pant and Nautial (1963) considered C. siamensis end C. pectinata similar on the basis of epidermal and anatomical studies.
Morphological Features of Cycas:
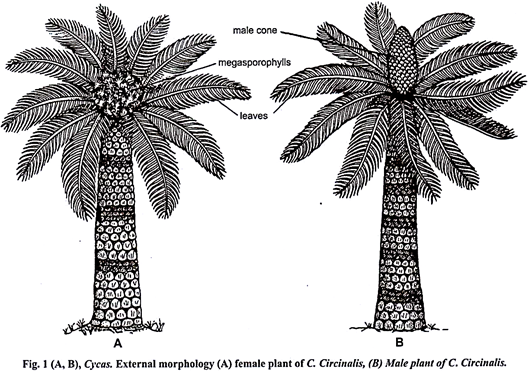
Cycas is perennial, slow growing evergreen plant and is referred as living fossil because it occurs as a fossil e.g., C. fusiana. It looks like a palm tree. Its main plant body is sporophytic, diploid, dominant and can be differentiated into three parts – roots, stem and leaves. Tallest species of Cycas is C. media with 20 feet height.
1. Roots:
They are of two types – normal and coralloid roots. Normal roots grow deep into the soil and form tap root system. Later it is replaced by adventitious roots. The function of these roots is to fix the plant in the soil and to absorb water and other minerals.
From the normal roots develop some small lateral apogeotropic branches near the ground surface. These lateral roots get infected with bacteria, fungi as well as algae. The entry of these organisms is said to be responsible for the characteristic, swollen, knob like or coral like appearance and hence, these roots are called as coralloid roots or corallorhiza. These roots have minute pores (lenticels like) which are respiratory in function (aeration). Root cap and root hairs are absent in coralloid roots (Fig. 2).
2. Stem:
It is thick, erect, woody, aerial and usually unbranched (caudex). Branching is rare and it is due to injury or development of adventitious buds. Surface of the stem is rough due to the presence of persistent woody leaf bases (Fig. 4). These leaf bases form thick armour around the stem.
In the armour are distinctly visible the alternating bands of large and small rhomboidal leaf bases. Larger ones are of foliage leaves and smaller ones are of scaly leaves and megasporophylls in the female plant. The leaf bases are spirally and compactly arranged with each other (Fig. 4).
At the top is present a crown of leaves (Fig. 1).
3. Leaves:
Leaves are dimorphic i.e., of two types – scale leaves and foliage leaves. Both these types of leaves form a crown at the top of the stem.
(a) Scale leaves:
These are small, dry, brown, triangular structures with a thick covering of brown hairs or rameta. These leaves alternate with green foliage leaves. These leaves protect the shoot apex and reproductive structures (Fig. 3).
(b) Foliage leaves:
These leaves are also produced in a crown at the apex of the stem. According to Coulter and Chamberlain (1910) one crown of foliage leaves is formed yearly while D.D. Pant (1953) observed the formation of two crowns per year in C. circinalis. In case of C. revoluta the leaves are 30 to 150 cm long but in case of C. circinalis they are up to 270 cm long.
A single foliage leaf is pinnately compound. It is unipinnate and paripinnate. Each leaf has 80-100 pairs of leaflets which are arranged on both the sides of adaxial groove of the rachis in opposite or alternate manner. The rachis is spiny below with the sheathing leaf base (Fig. 6A). these spines are modified leaflets. Each leaflet is leathery in texture, sessile elongated, ovate or lanceolate in shape and has entire margin with acute apex. Each pinna or leaflet contains a midrib without lateral veins.
In C. micholitzii the leaflet is repeatedly and deeply dichotomised (Fig. 5).
Margins of the pinnae are flat (Fig. 6B) but sometimes they are curved downwards and inwards (revolute) (Fig. 6C) which give the plant a specific name C. revoluta. Young leaves have circinately coiled leaflets which are also covered by hairs or ramenta like those of ferns (Fig. 6 D, E).
Internal Structure of Cycas:
1. Root:
(i) Normal root:
Its internal structure is exactly similar to that of dicot root. It is circular in outline and can be differentiated into epiblema, cortex and vascular tissue.
a. Epiblema:
It is the outermost limiting layer and consists of single layer of thin walled cells. Some of its cells give rise to root hairs.
b. Cortex:
Epiblema surrounds the multilayered zone of thin walled parenchymatous cortex with numerous intercellular spaces. The cells of the cortex are filled with starch. Some tannin cells, mucilage cells and sometimes sphaeraphides (calcium oxalate crystals) are also present in the cortex. The innermost layer of the cortex forms the endodermis which is characterised by the presence of casparian strips.
c. Vascular tissue:
Endordermis is followed by multilayered parenchymatous pericycle. Vascular bundles are radial. Xylem is diarch and exarch i. e., protoxylem is towards the periphery). The protoxylem consists of spiral tracheids whereas the metaxylem consists of scalariform thickenings. Vessels are absent. Alternating with the protoxylem groups are present phloem cells consisting of sieve tubes and phloem parenchyma. The companion cells are completely absent (Fig. 7A, B).
Secondary Growth:
The mature normal root shows secondary growth on both the lateral sides of primary xylem. Along with the inner side of primary phloem develops the cambium. It cuts off secondary phloem on outer side and secondary xylem on the inner side. After sometime the cells of the pericycle opposite to the protoxylem strands also become meristematic and behave as cambium, cutting phloem on the outer side and xylem on the inner side.
Thus, a complete ring of cambium is formed which forms a complete ring of secondary xylem on the inner side and complete ring of secondary phloem on the outer side. The primary phloem is crushed in the due course of development and appears in the form of crushed layer above the secondary phloem.
Simultaneously the formation of periderm also starts. The cells of the outermost layer of the cortex become meristematic (also called cork cambium) and start cutting cork cells on the outer side and secondary cortex on the inner side. In the course of the formation of cork, the cells of the epiblema are crushed (Fig. 8 A, B)
(ii) Coralloid Root:
The transverse section of the coralloid root is similar to that of normal root and it can be differentiated into epidermis, cortex and vascular tissue.
a. Epidermis:
In young root, it is similar to normal root. However, in old root the outermost tissue is periderm. It consists of 2 to 5 layers of dead cells.
b. Cortex:
The cortex is wider in comparison with the normal root. A greenish algal zone is present almost in the middle of the cortex and divides it into outer cortex and inner cortex (Fig. 9A, B).
The algal zone consists of loosely connected, radially elongated thin walled cells occupied by blue green algae (Anabaena cycadae, Nostoc punctiforme, Oscillatoria), bacteria (Azotobacter, Pseudomonas radicicola) and some fungi. The main function of these roots is nitrogen fixation due to the presence of cyanophycean members. Endodermis is similar to normal root.
c. Vascular tissue:
Endodermis is followed by multilayered parenchymatous pericycle. Vascular bundles are radial. Xylem is triarch and exarch.
Secondary growth is very rare or absent. No secondary xylem or secondary phloem are developed although cork and cork cambium are present.
Comparison between Normal Root and Coralloid Root:
Normal root:
1. Develops from the radicle, tap root system
2. Geotropic
3. Such characters are absent
4. Such infection is absent
5. Root hairs are present
6. Cortex is smaller
7. Such division is absent
8. Diarch
9. Secondary growth present
10. Main functions are: fixation of plant, absorption of water and mineral nutrients
Coralloid root:
1. Develops from the normal roots
2. Apogeotropic
3. Develops from the normal roots
4. Dichotomously branched and appears like coral
5. Gets infected with algae, bacteria and fungi
6. Absent
7. Cortex is wider in comparison
8. Due to presence of the algal zone in the cortex, it is differentiated into outer cortex and inner cortex
9. Very little or absent
10. Main function is nitrogen fixation
2. Stem:
A transverse section of young stem is similar to dicot stem. It is irregular in outline due to persistent leaf bases. Internally, it can be differentiated into epidermis, cortex and vascular cylinder.
a. Epidermis:
It is the outermost layer of the stem. It is made up of compactly arranged thick walled cells. Epidermis is ruptured due to the armour of persistent leaf bases (Fig. 11A).
b. Cortex:
Epidermis encloses the cortex. It forms the major portion of the stem. It is composed of parenchymatous cells which are filled with large number of starch grains. These starch grains are the source of sago starch. Therefore, C. revoluta is popularly known as sago palm.
Scattered in the cortex are various mucilage canals. Each mucilage canal is lined by many radially elongated epithelial or secretory cells (Fig. 10). which secrete mucilage. These canals are connected with those of the pith with the help of the medullary rays. The innermost layer of cortex is endodermis. It is not distinct.
c. Vascular Cylinder:
The vascular cylinder is surrounded by not very conspicuous pericycle. Like dicot stems vascular cylinder consists of many conjoint, collateral, open, endarch vascular bundles arranged in a ring (ectophloic slphonostele). The xylem consists of tracheids and Xylem parenchyma (Fig. 11B).
Vessels are absent. Outside the xylem is the phloem which consists of sieve tubes and phloem paraenchyma. Companion cells are absent. The Xylem is separated from the phloem with the help of primary combium. The cells of the primary cambium are brick shaped.
The cells lying in between the vascular bundles form the medullary rays. These are parenchymatous and connect the pith with the cortex. Each medullary ray is one celled wide and 1 to 20 cells long.
3. Pith:
In the centre of the stem is present large canals leaf traces massive pith consisting of parenchymatous cells which are rich in starch (sago starch). A large number of mucilage canals are also present, which are exactly similar in structure with the mucilage canals present in the cortex.
4. Leaf Traces and Girdle Traces:
The leaf traces are scattered in the cortex of the stem and constitute the vascular tissue of the leaves from the main vascular cylinder. Each leaf receives four traces, two of which are direct traces and the other two are given out from the opposite side of the direct traces.
These two traces take a round around the main vascular cylinder in opposite direction through cortex and then enter the leaf base of opposite side from the point of their origin from the stele. These leaf traces are known as girdle traces or indirect trances and are peculiar structures in the stem of Cycas.
Secondary growth:
It is a slow process. At first a complete ring of cambium is formed by the development of interfascicular combium in between the adjacent vascular bundles. The cambium cuts off secondary xylem on the inner side and secondary phloem on the outer side.
Tracheids consist of multiseriate bordered pits. This cambial ring is short-lived and new cambial ring is formed every year in the pericycle of the cortex. Wood formed by this method (more than one) cambium ring is polyxylic and manoxylic (large amount of parenchyma is cut off in the xylem. (Fig. 13).
5. Rachis:
A transverse section of the rachis is somewhat rhomboidal in outline, but a little higher up it is shield shaped. Its internal structure can be differentiated into epidermis, cortex and Vascular bundles.
a. Epidermis:
It is the outermost covering. It is made up of compactly arranged thick walled cells. It is single layered, covered with thick cuticle and has stomata.
Hypodermis:
Epidermis is followed by hypodermis. It is differentiated into outer 2-3- layers of chlorenchyma (Chlorophyll containing thin walled cells) and inner 4-6 layers of sclerenchyma (thick walled, lignified cells; Fig. 14A, B).
Ground tissue:
Below the sclerenchyma is present a large tissue made up of thin walled parenchymatous cells. It is called ground tissue. In this region are present many mucilaginous canals and vascular bundles.
b. Vascular bundles:
Vascular bundles are arranged in the shape of inverted Greek letter ‘omega’ [Ω; Fig. 14 A], Each vascular bundle is conjoint, collateral, endarch, open and diploxylic i. e., consists of centripetal and centrifugal Xylem and is surrounded by bundle sheath.
Xylem is present towards the inner side and consists of tracheids and xylem parenchyma. Vessels are absent. Phloem is present towards the outer side of the vascular bundle. It consists of sieve tubes and phloem parenchyma. Companion cells are absent, Cambium is present in between the xylem and phloem.
In rachis the vascular bundles are endarch at the base (centrifugal xylem is well developed, protoxylem faces towards the centre showing endarch condition, centripetal xylem is not developed), mesarch in the middle (centripetal and centrifugal xylem are present showing diploxylic condition) and exarch at the apex (centripetal xylem is well developed, triangular and exarch, centrifugal xylem is much reduced and in the form of two patches lying one on each side of the protoxylem elements of centripetal xylem) due to twisting of the rachis (Fig. 15 A-C).
6. Leaflet:
The leaflet of Cycas is dorsiventral and hypostomatic (the stomata are present at the lower surface only). In a transverse section the leaflet can be differentiated into a swollen midrib portion and two lateral wings (Fig. 16A, B).
Its internal structure is as follows:
a. Epidermis:
It is the outer most single layer made up of squarish cells. The upper epidermis is complete whereas the lower epidermis is interrupted by several sunken stomata present in the region of the wings. The upper and lower epidermis is covered by a thick layer of culicle.
b. Hypodermis:
Below the epidermis occurs the thick walled sclerenchymatous hypodermis. It is single layered in the region of blade but in the region of mid rib it becomes 2-3 layered thick. Two to five layers of sclerenchymatous cells are also present above the lower epidermis only in the region of the mid rib. It helps in checking the rate of transpiration and protects the tissue from excessive heat.
c. Mesophyll:
A well-developed mesophyll tissue is present in the leaflet. It is differentiated into palisade tissue and spongy parenchyma. Palisade tissue is present in the form of continuous layer below the sclerenchymatous hypodermis. Spongy parenchyma present only in the wings directly above the lower epidermis. It is made up of loosely arranged oval cells filled with chloroplast. These cells have many intercellular spaces filled with air.
d. Vascular bundle:
A single large vascular bundle is present in the mid rib region of the leaflet. It is surrounded by a single layer of sclerenchymatous cells, known as bundle sheath. The vascular bundle is conjoint, collateral, open and diploxylic. Xylem is present towards the dorsal surface and phloem is present towards the ventral surface.
Xylem and phloem are separated by a non-functional strip of cambium. Centrifugal xylem is represented by two small groups on either side of the protoxylem. The remaining space of the vascular bundle is filled with thin walled parenchymatous cells.
e. Transfusion tissue:
Groups of tracheidal cells, separated by some parenchymatous cells, or directly in contact with the centripetal xylem, the bundle sheath are present in the leaflet. It is called primary transfusion tissue. The cells of this tissue are short and wide with are reticulate or bordered pitted walls.
A zone is present on either side of the midrib between the palisade and spongy layers. It is three layered and is composed of elongated colourless cells. These cells run paralled to the leaf surface from the midrib to the margin. This zone is called accessory transfusion tissue or secondary transfusion tissue or hydrostereom or radial parenchyma.
On either side of the leaflet it is connected with the primary transfusion tissue present around centripetal xylem of the vascular bundle. Primary and secondary transfusion tissue help in the lateral conduction of water. The presence of transfusion tissue is to compensate for the unbranched condition of the midrib and it probably serves as a later conducting channel of water.
Reproduction in Cycas:
Cycas reproduction by two method – Vegetative and Sexual
1. Vegetative reproduction:
It is the simplest method of reproduction. It takes place by the formation of bulbils or adventitious buds. These buds develop on the stem in the axil of the scale leaves. A bulbil is an oval structure, broad at the base and pointed at the apex. It consists of dormant stem in the centre covered by numerous brown scaly leaves.
On detachment from the stem, a bulbil starts to germinate by producing many roots from the lower side and a leaf towards the upper side. A bulbil from male plant will develop only into male plant while the bulbil from the female plant will form only female plant because cyas is strictly dioecious (Fig. 1A, B).
2. Sexual Reproduction:
Sexual reproduction in cycas is oogamous (the female gamete i.e., egg cell is significantly larger than the male gamete and is non-motile). Cycas is sporophytic and strictly dioecious i.e., male and female sex organs are borne on separate plants.
Male Reproductive Organs:
Male plant of cycas produces every year a single male cone (Fig. 1B) at its apex. In the formation of the male cone the apical meristem is used up, and therefore, the growth of the steam checked for some time some time but later an apical meristem is formed at the base of the cone, which pushes that on one side so that the growth of the stem is resumed again.
Such growth of the stem is called sympodial (Fig. 18 A, B). The male cone is largest in the plant kingdom (approximately 500 cm or more in length).
Longitudinal Section of Male Cone:
Each cone is an ovoid or conical structure (Fig. 19A). A longitudinal section of male cone shows that each one consists of a central axis around which, a large number of leaf like structures called as microsporophylls are attached at right angle in a compact, spiral, acropetal succession (Fig. 19.B). The maturation of the sporophylls takes place in a spiral manner i.e., from apex to base. However, a few sporophlills at the apex and base remain sterile. Fig. 19 B.
Structure of microsporophyll:
Each microsporophyll represents a stamen. It is a flattened, woody and triangular structure. It is differentiated into upper or distal, sterile region called apophysis (Fig. 20 A) and proximal wedge shaped fertile part. Each microsporophyll bears several hundred microsporangia (pollen sacs) on its abaxial surface (more than 1000, Fig. 20B-D).
Microsporangia are arranged in clusters of 3 to 6. Each cluster or group of microsporangia is called sorus. In between the microsporangia indusial hairs are present which help in the dispersal of the microspores and protect young sporangia.
Development of Microsporangium:
The development of microsporangium is of eusporangiate type i. e., develops from a group of cells called microsporangial initials (Fig. 21A). These cells are hypodermal in origin and divide by periclinal walls into upper primary wall cells and lower primary sporogenous cells.
Primary wall cells divide and redivide to form three to six layered wall below the epidermis. Simultaneously primary sporogenous cells also divide and redivide irregularly to form a mass of cells known as sporogenous tissue.
At this time from the peripheral cells of the sporogenous tissue or the inner most wall layer differentiates into a single celled, nourishing layer known as tapetum (Fig. 21C, D). The outermost layer of the sporangial wall forms the epidermis or exothecium.
The rest of the sporogenous tissue increases in size and functions as spore mother cells. These are the ultimate cells of sporophytic phase. Each spore mother divides cell by reduction division to form four haploid microspores (Fig. 21D-F).
A mature microsporangium is sessile or shortly stalked consists 5-6 wall layers (outer single layer exothecium, innermost single layer tapetum and rest endothecial layers). The wall of sporangium encloses large number of haploid microspores. The tapetum is used up during the development of microspores. Only the signs of disintegrated tapetum can be seen at maturity.
Female Reproductive Organs:
Female reproductive organs are megasporophylls. Each female plant every year produces numerous megasporophylls in acropetal succession above each crown of foliage and scaly leaves. There is no female cone formation. The number of the megasporophylls is much more than the number of the foliage leaves on the stem.
During the formation of the megasporophylls the apical meristem is not used up like that of male cone and therefore, the growth of the stem continues, and thus in female plant growth is monopodial.
Structure of Megasporophyll:
Each megasporophyll (carpel) is regarded as a modified leaf. It is about 12.7 cm to 25.4 cm long and can be divided into 3 parts: upper leafy portion, middle ovule bearing portion and lower stalk. Ovules are formed on the lateral side of the middle portion. The upper portion is pinnate and each pinna is tapering to a point.
Two lateral rows of ovules are present on the lateral side of the middle portion. In Cycas there is a great variation regarding the pinnate character of megasporophyll and the number of ovules per sporophyll as a result of which in various species of Cycas gradual reduction in megasporophylls can be traced.
The megasporophylls of C. revolula (Fig. 22A) are pinnate whereas those of C. circinalis C. rumphii and C. beddomei (fig 22 B-D) are ovate lanceolate structures. In C. pectinata and C. siamensis they are orbicular or rhomboidal structures (Fig. 22B, F).
The laminar portion is well developed in C. revoluta, C.pectinata and C. siamensis but reduced in C. circinalis, C. beddomei and C. rumphii (fig. 22). The margin of lamina is serrate or dentate in C.circinalis, C.beddomei and C. rumphii. The number of ovules differ in different species of Cycas. It is 1-6. pairs in C.revoluta, C. Circinalis and only one pair in C. norambyana. Megasporophylls are covered by yellow or brown hairs.
Structure of ovule (megasporangium):
The ovules are sessile and are borne laterally on the stalk. The ovules of Cycas are largest in plant kingdom (7 cm long in C. thoursaii, 6 cm long x 4 cm diameter in C. circinalis) and can be seen by naked eye. The ovule is green when young and is covered by hairs. At maturity its colour changes to orange and hair also fall off.
The ovules are orthotropus (short and straight) and unitegmic (with one integument).
The integument is very thick and consists of three distinct layers:
(i) Outer, green or orange fleshy layer called outer sarcotesta
(ii) Middle, yellow stony layer called sclerotesta and
(iii) Inner fleshy layer or inner sarcotesta.
The parenchymatous tissue inside the integument is called nucellus. The integument encloses all the nuclellus except at one point. This point or opening is called micropyle. Just below the micropyle, the cells of the nucellus form the nucellar beak.
Some of the cells of the nucellar beak dissolves and forms a cavity like structure called pollen chamber. Just below the pollen chamber is present an archegonial chamber. Micropyle leads into the pollen chamber. Just below the floor of the archegonial chamber 3-6 archegonia are present towards the micropylar end.
The ovule is supplied by three vasular traces (Fig. 23). The central vascular trace enters the chalazal end of the nucellus. The inner and outer vascular traces divide into two each, one branch supplies the outer fleshy layer and the inner fleshy layer. Thus, the outer and inner fleshy layers receive the vascular supply but the middle stony layers get no vascular supply (Fig. 23).
Development of Ovule:
When megasporophyll is young; in its middle portion 4-6 ovules arise as a hypodermal mass of meristematic cells on the lateral side. These meristematic cells divide and redivide to form a mass of parenchymatous cells known as nucellus.
Soon the neighbouring cells at the base are also activated and they grew upwards forming the integument which surrounds the nucellus on all sides except at the top where a small opening is left which is known as micropyle (Fig. 24A, B). In the beginning the nucellus and integument are free but afterwards due to intercalary growth both of them fuse except in the region of micropyle.
Deeply situated in the nucellus, any one cell enlarges and functions as megaspore mother cell or embryo sac cell. It divides by meiosis to form a linear tetrad of four megaspores. Upper three degenerate (Fig. 24 C-F) and lower most is functional. It is the first cell of female gametophyte.
Gametophytic Phase:
Microspores are formed in microsporangia and megaspores are formed in ovules after meiosis. Cycas is heterosporous because it produces two types of spores.
Development of male gametophyte:
Each microspore is unicellular, unicelled structure with two layered wall. Outer wall is known as exine while inner is known as intine.
The development of male gametophyte takes place in two stages:
Stage I:
Development of male gametophyte before pollination: Development of male gametopheyte or germination of pollen grains starts in situ i. e., they are still inside the microsporangium.
First its nucleus divides into two, one of them goes towards the lower side and is separated from the other by a cresent shaped wall resulting in the formation of two unequal cells. The lower smaller cell is called as prothallial cell and the upper bigger one as antheridial cell (Fig. 25 A, B).
The prothallial cell does not divide further but the antheridial cell divides to form a generative cell (Fig. 25C) which is in close contact with the prothallial cell and distal tube cell with a large nucleus. This stage is called 3-celled stage consisting of a prothallial cell, generative cell and tube cell.
At this stage microsporangia dehisce and the shedding of the pollen grains takes place, (tapetum disintegrates, sporangium becomes dry, cells shrink, sporangial wall ruptures radially at the line of dehiscence). Further development of the pollen grains (II stage) takes place after pollination.
Development of Female Gametophyte:
The functional megospore (also called embryo sac cell or first cell of female gametophyte) is haploid and it starts its development in situ i. e., within the nucellus (Fig 26 A). It absorbs the surrounding cells of the nucellus and enlarges considerably. Its nucleus divides by free nuclear divisions and as a result a large number of nuclei are formed. A vacuole develops in the centre.
It pushes the free nuclei and cytoplasm of the megaspores towards its periphery. (Fig. 26 B) Now the wall formation starts from periphery to the centre (centripetal wall formation). It results in the formation of a cellular tissue called endosperm or female prothallus or megagametophyte (Fig. 26 C). The endosperm in Cycas is a haploid tissue formed before the fertilization.
Endosperm is nutritive in function. Simultaneously, a tiny space develops on the upper side of the ovule between nucellus and the female gametophyte due to degeneration of certain nucellar cells. This is called archegonial chamber.
Development of archegonium:
The archegonia develop from the gemetophytic cells lining the archegonial chamber towards the micropylar end. Any cell enlarges in size and functions as archegonial initial (Fig. 27A). It divides transversely into an upper primary neck cell and a lower central cell (Fig. 27 B). The primary neck cell divides by a longitudinal division to form two neck cells. These cells form the neck of the archegonium.
The central cell enlarges its size and its nucleus divides to form a ventral canal nucleus and an egg nucleus (Fig.27 C). No wall is formed between the venter canal nucleus and egg nucleus. Therefore, there is no neck canal cell. Later on venter canal nucleus disorganises. The egg of the Cycas is largest in all living plants measuring 5 mm in diameter.
Structure of Archegonium:
The mature archegonium consists of a neck of two neck cells. The archegonial neck opens in the archegonial chamber. There is no neck canal cell. There is no venter either. The egg and the venter canal nucleus remain surrounded by the cells of the endosperm. These cells act as archegonium jacket.
Pollination:
The pollination is anemophilous. The cells of nucellar beak present in the pollen chamber disintegrate and form a viscous fluid. This fluid is cohesive in nature. This fluid oozes out of the micropyle and collects in the form of a pollination drop.
The pollen grains present in the air current at their 3-celled stage, are entangled in the pollination drop. Gradually the pollen drop dries up and the pollen grains are sucked into the pollen chamber through micropyle. Further drying of this drop seals up the micropyle (Fig. 28) Pollen drop helps in collecting the pollen grains at the micropyle in all gymnosperms.
II Stage. Development of male gametophyte (after pollination):
After a definite period of rest (pollen grains may lie inside the pollen chamber for quite some time say for four months, the interval between pollination and fertilization is about 20 days in C. revoluta). Further development of the male gametophyte takes place in the pollen chamber. The exine breaks up and intine comes out in the form of pollen tube.
It may be branched or unbranched and acts as an absorbing organ (Pollen tube acts only as an absorbing organ or hustorium because the sperms are formed only after the complete development of the pollen tube) Pollen tube, penetrates the nucellar tissue and comes to lie in the archegonial chamber. The generative cell divides into a lower stalk cell and upper body cell (Fig. 25D). The stalk cell does not divide further. The body cell divides at the time – micropyle of fertilization.
Now two blepharoplasts appear, one at each pole of the nucleus of the body cell in a transverse position. Body cell divides longitudinally into two sperm cells, each of these having a single nucleus, blepharoplast and a small amount of cytoplasm. Each sperm cell later on develops into a sperm and blepharoplast gives rise to cilia. The sperms are liberated in the pollen tube by the breaking of the sperm mother cell (Fig. 25E, F).
The sperms of the Cycas are largest in the plant kingdom (180-210 µ) and visible to the naked eye. Each sperm is more or less triangular, top shaped mobile structure having five to six spiral bands with thousands of cilia with a single large nucleus. By means of their cilia, they move freely in the pollen tube (Fig. 25F).
Fertilization:
At the time of fertilization, the nucellar tissue between the pollen chamber and the archegonial chamber disorganise and simultaneously the venter canal nucleus also disintigrates. The pollen tube reaches the archegonial chamber (Fig. 29). The tip of the pollen tube ruptures releasing two male gametes and fluid contents.
Due to this archegonial chamber becomes moist and the sperms move freely in it with the help of cilia. Only a single sperm enters violently in each archegonium through neck. Only the male nucleus of the sperm fuses with the egg nucleus to form a zygote or oospore (2x). The fertilization in Cycas takes place with the help of motile sperms.
This process is known as zooidogamy. It is accompanied by pollen tube formation, a phenomenon known as siphonogamy. Sometimes more than one sperm enter the archegonium but the male nucleus which first reaches near the nucleus fertilizes the egg. Rest male nuclei degenerate. It is called polyspermy.
Embryogeny:
The fertilized egg, zygote or oospore is the first cell of the sporophyte. The zygote contains dense cytoplasm and a large nucleus. It enlarges in size and finally forms the embryo. In this whole process one year time is utilised. The nucleus moves at the base and starts dividing by free nuclear divisions to form about 256 free nuclei (Fig. 30A, B).
The nuclei and cytoplasm of the central region disorganise forming a central vacuole (Fig. 30C). Subsequently wall formation takes place from the base and advances towards the upper side forming a small mass of cells. This embryonal mass of cells is called proembryo (Fig. 30D). It is meristematic in nature. Some of the nuclei in the upper region remain without cell walls.
The proembryo soon gets differentiated into three zones (Fig. 30E):
(1) Upper haustorial zone at the micropylar end
(2) The middle suspensor zone
(3) Tha basal embryonal zone.
The haustorial zone absorbs the nutrition for the developing embryo. Suspensor zone elongates considerably forming a long, spirally coiled suspensor, which pushes the embryo in the food containing cells of the endosperm. The embryonal zone is differentiated into embryo. It is differentiated into two cotyledons (three in C. circinalis).
A plumule is then differentiated in between the cotyledonary depression. The embryonal part lying below the cotyledonary attachment is the hycotyl. A radicle is now differentiated at the base of the hypocotyl. It is protected by a hard pad like protective covering called coleorhiza. It provides protection to the radicle (Fig. 30 F, G).
Several archegonia may be fertilized in an ovule of Cycas. It results in the formation of several zygotes. These zygotes may undergo initial development but usually one embryo reaches maturity.
Structure of seed:
After fertilization, the ovule is transformed into seed. The nucellus and the inner layer of integument are used up as nourishment by developing embryo. The mature seed appears as orange-red or reddish brown structure.
It comprises the following structures:
a. Testa or seed coat:
It is formed by the outer brightly coloured fleshy layer and the middle layer of the integuments.
b. Micropyle:
It is present in the form of small opening at the top of the seed.
c. Endosperm:
Inner to the seed coat lie the wall tissues called endosperm. The cells store a large amount of food material.
d. Embryo:
Embedded in the endosperm lies the embryo. It consists of two cotyledons, plumule and radicle. The embryo remains suspended in the endosperm by a long spirally coiled suspensor (Fig. 32).
Thus, a mature seed of Cycas represents three generations:
Seed Coat:
It is formed by the integument and represents parent-sporophytic generation.
Endosperm:
Represents the gametophytic generation.
Embryo:
It represents the new sporophytic generation.
Testa is sweet in taste and emits pleasant odour. The two characteristics i. e., red colour and pleasant odour are responsible for their zoochorus (orinthochorous) dispersal.
Germination of seed:
Seeds remain viable for not more than a few months. Under suitable conditions the seed starts germination. It absorbs water and embryo expands. The expansion of the embryo breaks open the hard seed coat.
The coleorhiza protrudes out and is pierced by the growing radicle which grows down and forms the tap root or primary root (Fig. 32A). The cotyledons do not come out of the seed coat but they absorb food from the endosperm for the growing embryo.
The stalk of the cotyledons elongates to carry the plumule out of the seed coat. The growing plumule at first, forms a few scaly leaves and then foliage leaves. The young foliage leaves show circinate vernation (Fig. 32 B-D).
The seed germination is hypogeal because the cotyledons remain underground enclosed in the female prothallus or endosperm. However, according to some workers cotyledons do not come out of the seed during germination as they are haustorial in nature, but their bases remain exposed, hence the seed germination may be said to epigeal.
Economic Importance of Cycas:
1. Several species of Cycas of e.g., C. revoluta, C. circinalis C. bedomei are grown in the gardens as ornamental plants.
2. From the stem ‘sago’ a kind of starch, is obtained. Hence, it is also called sago palm (C. revoluta)
3. Leaves of C. circinalis are used to prepare hats, baskets and mats. The leaves of Cycas are extensively used for decorative purposes and floral decoration.
4. The resin obtained from C. rumphii is applied to malignant ulcers.
5. Juice of tender leaves of Cycas is useful for vomiting and flatulence (the presence of excessive gas in the digestive tract).
6. Pollens of C. circivalis are narcotic. Its seeds ground to paste with coconut oil are useful for sores and swellings.
7. Seeds of Cycas are roasted and are used as food in Assam and certain islands.
8. The seeds and stem of C. revoluta are used in making wire in Japan.
9. The flour of seeds of Cycas is called Indum Podi and is used in the preparation of cakes and porridges.
10. The young succulent leaves of Cycas are also cooked as vegetable.