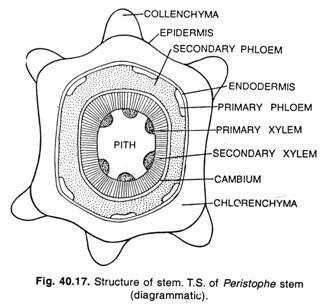
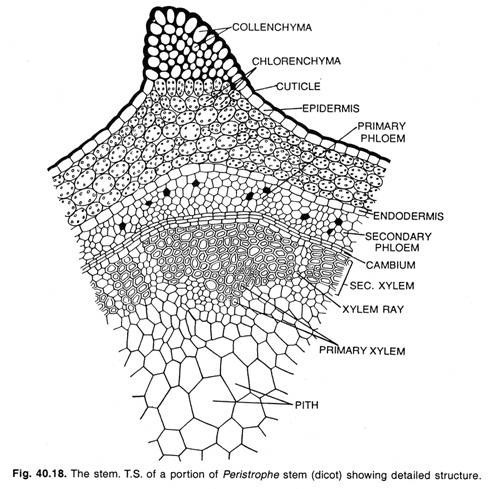
Here is a compilation of essays on ‘Reproductive Cycles in Vertebrates’ for class 9, 10, 11 and 12. Find paragraphs, long and short essays on ‘Reproductive Cycles in Vertebrates’ especially written for school and college students.
Contents
Reproductive Cycles in Vertebrates
Essay Contents:
- Essay on the Reproductive Cycles in Marine Fishes
- Essay on the Reproductive Cycles in Freshwater Fishes
- Essay on the Reproductive Cycles in Birds
- Essay on the Reproductive Cycles of Lizards
- Essay on the Reproductive Cycles of Frogs
Essay # 1. Reproductive Cycles in Marine Fishes
Male Reproductive Organs:
A pair of testes is situated in the abdominal cavity, one on either side of the kidneys below the air bladder. These are enlarged and flattened structures suspended lengthwise by mesenteries (mesarchia). They may or may not be equal in size. Each testis is composed of follicles in which the spermatozoa formed make their way towards the exterior through the coiled tube, vasa efferentia.
These open into the anterior end of the vas deferens. The vas deferens becomes enlarged to form the sac like seminal vesicles on each side before they open into a large triangular chamber, the urinogenital sinus, which finally opens into the cloaca on an elevated urinogenital papilla.
In bony fishes, each testis possesses a sperm duct in its posterior side and the two sperm ducts join posteriorly to open to the exterior through a common urinogenital pore placed behind the anus. Most often the testes are creamy white in color and smooth. The contents are granular.
In the earlier stages, testes are thread like, becoming thick and fleshy with age and maturity. In mature specimens, the testes occupy almost the entire length of the abdominal cavity and are rose red in color with ridges and furrows, which apparently divide them into a number of lobes.
The ovaries in majority of marine fishes are paired, elongated, sac-like structures extending lengthwise in the abdominal cavity, ventral to the kidneys. They are attached to the body wall by means of the mesovaria. In elesmobranchs, the oviduct with a funnel at its head end is situated anteriorly in the body cavity and conducts the eggs caudally to their cloacal exit.
In oviparous elasmobranchs, oviduct is modified anteriorly into a shell gland, while in ovoviviparous and viviparous fishes; it is enlarged to form uterus for retention of embryos during their development. In bony fishes, the anterior ends of the two ovaries are free but their caudal ends may become united into one.
The posterior end of each ovary continues into a short oviduct. The two oviducts open together to the exterior through a genital aperture. Generally both the ovaries are equal in size but occasionally they are unequal.
The paired ovaries vary greatly in their appearance, size and general structures during the growth. The size and extent of the ovaries in the body cavity vary with the stage of sexual maturity. The color varies from whitish in young through greenish when immature to golden yellow in ripe adults. Texture of the ovaries ranges from floccular to granular in adults.
Regarding maturity stages, the vast majority of fishes show cyclic or periodic reproductive behaviour. For the determination of maturity cycle, the most common method is to define the stage of maturity of the gonad. The International Council for the Exploration of the Seas (ICES) has recognized seven maturity stages, 1st and 2nd as immature, 3rd and 4th as maturing, 5th as mature, 6th as ripe and 7th as spent.
Earlier workers used different features of the gonads as the bases of classification of the maturity stages involving morphological and histological examination of the gonads of the fish under study. Morphological observations like color, shape and size of the gonads in relation to the body cavity were used for ascertaining the maturity stages.
Microscopic examination of gonadal products, covering various histological methods over a year can give an idea about the maturation cycle. In case of female fish, ova diameter frequency helps in predicting some of the events in the maturation cycle. In the first classification it is based on the size of the ova but later the diameter of the ova was taken into consideration.
Maturation and Spawning:
Development of Ova to Maturity:
The maturation cycle in a fish is largely dependent of the growth rate of the ova of different stages in the ovary and subsequently on their distribution in the mature ovaries. Maturation refers to cyclic morphological changes, which the male and female gonads undergo to attain full growth and ripeness. The definition does not include the complicated physiological changes involving endocrine control.
The following classification traces the development of ova from immature to ripe condition:
(a) Immature Ova:
These include the minute transparent ova as they arise from the germ cells, from the time they could be distinctly recognized as possessing a nucleus and a protoplasmic layer.
(b) Maturing Ova:
These include all the small opaque ova in which the formation of yolk has just commenced, but which are not fully filled with yolk.
(c) Mature Ova:
This group includes all the ova that are opaque, full of yolk and with distinct yolk spherules, but still contained within the follicle.
(d) Ripe Ova:
Include all those fully mature large, free, fully or partly transparent eggs, which have burst from the follicles.
The common method followed by most workers is the ova diameter frequency distribution that gives an index of the progression of oocytes and their withdrawal from the ovaries.
Spawning Season:
Spawning season indicates the range and peak of maturity with respect to time. Spawning in teleost fishes occurs during a particular phase of reproductive cycle. Some breed once in a year (annual), some breed throughout the year at regular intervals and in some, such as Pacific salmon, death follows spawning. This implies that a sound Knowledge of the reproductive cycle of a species is essential in fisheries management and rational exploitation.
The spawning season can be determined by the following methods:
a. Occurrence of Mature Fishes:
In this method, observations carried out for at least a year are analyzed and their percentages calculated month-wise. Spawning season is determined on the basis of the distribution of the different maturity stages, particularly the dominance of advanced stages with respect to time.
b. Gonado-Somatic Index (GSI):
Gonado- somatic index is another method for studying the spawning season by following the seasonal changes in the gonad weight in relation to body weight.
c. Occurrence of Eggs and Larvae:
The variation in the peak spawning period is dependent on the availability of favorable environmental conditions. The spawning season extends from May to August with maximum activity during June and July. Spawning takes place at mid night or early morning. The breeding is in relatively shallow in shore waters and there is only one spawning season for the fish during its lifetime.
Spawning Periodicity:
In temperate as well as tropical waters, fishes exhibit different spawning periodicities, which are closely related with the development of eggs, thus establishing a close relationship between the structure of the ovary and the spawning behaviour. The length of the breeding period is extremely variable and some species may spawn only once, others twice, while still others several times in a year.
The spawning activity in fishes can be divided into four types:
Type A:
Spawning occurs only once in a season during a short period of time. In the ovaries of fishes which spawn only once in a season and in which the duration of spawning is restricted to a definite and short period, the mature stock of ova are found differentiated from the general egg stock. In the whole ovary, only one batch of mature eggs is found. They are shed during the succeeding spawning season.
Type B:
Spawning takes place only once a season but for a longer duration. In species showing this type of spawning, the range in size of the mature ova, irrespective of the number of modes representing them, has been found to be nearly half the total range in size of the entire eggs in the ovary.
Type C:
These fishes spawn twice a season. In the ovaries, in addition to the batch in ripe condition, another batch of eggs in which yolk formation has already commenced is usually present in the ovary. Such modes, representing ova half way to maturity have been found more or less in the middle of the total range in size of the eggs in the whole ovary.
Type D:
This group includes fishes breeding throughout the year but intermittently. Different batches of eggs in the ovary are not sharply differentiated from one another, indicating that the passing of one batch of eggs into the next stage is a continuous process.
Environmental Factors Controlling Gonadal Cycles:
The process of maturation of gonads in fishes is controlled by internal as well as external factors so that the individuals spawn together during favorable environmental conditions. Internal factors comprise a series of hormone- controlled changes occurring both in testes and ovaries in such a way that peak maturation is attained during a stipulated time.
In many fishes coordination in spawning is achieved by shoaling behaviours. Very often, the spawning congregation involves migration over long distances and this is so adjusted that large number of individuals are available at the spawning ground. Both internal and environmental factors are responsible for the behaviours leading to congregation, while the fish is shoaling; the contents of both male and female gonads are released to the exterior where fertilization takes palace.
The process of fertilization is epidemic in nature as spawning by one individual induces the others to follow the same. Males reach the spawning grounds before the females and the presence of spermatozoa induces the females to shed their eggs. The internal physiological rhythm of gonadal maturation involving pituitary and gonadal interactions is adjusted to ensure that breeding occurs under most favorable environmental conditions for survival of the offspring.
Many environmental factors affect different phases of the breeding cycles. The proximate factors include light, temperature, and other physical factors while the ultimate factors are food availability and favorable growth conditions. Photoperiod also plays an important role.
Spawning period of tropical fishes is correlated with water characteristics such as temperature, salinity, wind and rain, which have a derisive influence on the beginning of the spawning season. These monsoon changes cause intermixing or upwelling of the hydrological factors. Temperature and salinity exert profound influence on the fish throughout its life cycle.
Temperature controls the metabolic and spawning activities while salinity not only exerts effect on spawning but also on osmotic balance of the body fluids of fishes. Optimum temperature for each species varies but generally lies between 17 to 24 °C. Favourable temperature and abundant food supply favor spawning in temperate countries but fail to trigger spawning in tropical waters.
Gonado-Somatic Index:
Gonads undergo regular seasonal cyclic changes in weight, particularly in females. Such cyclical changes are indicative of the spawning seasons. Therefore, the gonado-somatic index is considered to be a method for studying the spawning season. Relative ovary weight indicates the state of maturity of the ovary.
Fecundity:
Fecundity is the number of ova formed in a season. In some fish like the herring, the number of yolky eggs in the ovary in a season can be accurately counted and this gives the fecundity of the fish. However, in Indian fish this is not possible because eggs are released from the ovary in batches.
Essay # 2. Reproductive Cycles in Freshwater Fishes:
Structure of Testes:
Testes of teleosts are covered over by tunica albuginea, a thin delicate membrane enveloping fibrous connective tissue. Internally each testis is lobulated and possesses somatic and germ cells. The tunica albuginea remains thin but the underlying stroma undergoes changes with maturity cycle, being thickest in spent testes.
The connective tissue fibres from the peripheral stroma penetrate and divide the lumen of testes into large number of seminiferous tubules or lobules of vaied shapes and sizes. The seminiferous lobules or tubules undergo changes in accordance with maturity cycle. The gonocoel, comprising of inter-and intra-lobular portions, is packed with both germ and somatic cells.
Although the germ cells are restricted to intralobular region, the somatic cells are distributed both within and outside the lobules. The architectural combination of these cells is highly variable during different seasons in relation to maturity stages.
Germ Cells:
The various types of germ cells that can be seen within a testis during its maturity cycle are sperm mother cells (resting or primary spermatogonia), secondary spermatogonia, spermatocytes, spermatids and spermatozoa (Fig. 1).
Resting or Primary Spermatogonia or Sperm Mother Cells:
In size, these are the largest of germinal cells. They lie within the lobules close to the lobular wall. These cells are with clear eosinophilic cytoplasm and acentrally placed hyaline nuclei.
Secondary Spermatogonia:
These resemble primary spermatogonia except for being smaller in size and possession of chromatin threads in the nucleus, which radiate from nucleolus.
Spermatocytes:
These are of two types, primary and secondary and are more centripetally arranged in the lobules. Secondary spermatocytes are smaller in size but their cysts are comparatively larger. These possess hyaline cytoplasm and central chromatin material. Distinct nuclear membrane is often lacking.
Spermatids:
These are round, darkly stained cells usually filling the entire lobule. Each spermatid comprises of a prominent nucleus enclosed within a small layer of hyaline cytoplasm.
Spermatozoa:
These are derived from spermatids as a result of spermiogenesis and each sperm has an anterior darkly stained nucleus and a small posterior part drawn into a tail.
Somatic or Non-Germinal Elements:
Interstitial Tissue:
The testes of teleosts can be divided into two basic structural types; the first category includes fishes, which have testes with discrete interstitial cells between testicular lobules while the second category includes fishes with testes devoid of true interstitium, but characterized by possession of tubules with boundary cells.
Functionally, both these cell types are comparable with the Leydig cells of higher vertebrates. These cells secrete steroid hormones, which control development and maintenance of secondary sexual characters, and also the reproductive activity.
Phagocytes:
These are darkly stained eosinophilic cells with a well- defined nucleus and variable shapes. These make their appearance in the testes towards close of spawning season (in spent or partially spent testes) and grow in number and size; their size being maximum in a testis prior to initiation of its successive maturity cycle.
Migratory Cells:
Spindle shaped or oval migratory cells are present within the interlobular septa and tunica of the testes. These cells have been reported to play an important role in replenishment of new crop of germ cells. Based on the staining properties with Sudan black, and the changes in their shape and number, they are considered to be closely related to interstitial cells. In the majority of freshwater fishes ovaries are paired and elongated structures. They are placed along the ventro-lateral margins of the body cavity of the fish.
Structure of Ovary:
Ovaries in teleosts may be of gymnovarian or cystovarian type (Fig. 2A). Gymnovarian ovaries are naked since the investing cover, the tunic is absent and the ovulated eggs shed in coelom are carried by the oviducal funnels to the oviduct. In some fishes like salmon these ducts are absent.
The cystovarian ovaries, on the other hand, are closed by a fold of peritoneum the tynica, which continues posteriorlyl as oviducts. The tunic is made up of outer connective tissue and inner germinal epithelium. The germinal epithelium is projected into the ovarian lumen in the form of freely suspended folds.
Histologically ovary consists of developing oocytes in different stages (immature, maturing and mature) besides corpus luteum and corpus atreticum of ovulated and unvoulated follicles depending upon the stage of ovarian maturity. A mature oocyte is surrounded by three layers; the outer theca, middle follicular epithelium and innermost vitelline membrane.
The first two layers perform major functions in the transport of nourishment to the oocyte, resorption of corpus atreticum and formation of corpus luteum. Intestitial cells are the other histological elements observed in the ovaries in varying proportions at different times of the year. Theca, follicular epithelium, corpus atreticum, corpus luteum and interstitial cells are steroid hormone producing sites in the ovaries of different fishes.
Atretic Follicles:
The term corpora atretica refers to preovulating follicles turning atretic. The cells of egg membranes phagocytose the yolk in a developing ovum and ultimately degenerate. This process clears the ovary of debris of oocytes, which fail to mature.
The formation of corpus atreica occurs in four stages:
Stage I:
This stage is characterized by the breakdown of the ovum and hypertrophy of granulose cells.
Stage II:
Invasion of follicular space by granulose cells, fragmentation of vitelline membrance, and phagocytosis of yolk by granulose cells are the most conspicuous changes, which occur at this stage.
Stage III:
Complete occupation of oocytic space by hypertrophied cells and completion of phagocytosis of yolk takes place during this stage.
Stage IV:
All the above structures disintegrate and the vascular space is completely filled by cell masses. Yolk is completely absent in the atreticum.
Corpus Luteum:
In most of the freshwater fishes the follicular envelope after the release of the ovum is retained in the ovary. Such discharged follicles consist of granulose and theca, which lie exterior to vitelline membrane. The follicles hypertrophy through developmental stages to form a solid corpus luteum.
The formation of corpus luteum occurs in the following stages:
Stage 1:
After ovulation, the postovulatory follicle shows a large cavity surrounded by the hypertrophied granulose and thecal layers. The granulose cells are columnar in shape with basal spherical nuclei. The theaca becomes thicker and consists of fibrous elements and connective tissue. Some of the thecal cells appear glandular. The theca is highly vascular.
Stage 2:
In this stage the size of postovulatory follicles is reduced. The granulose layer forms villus-like projections, which are irregularly placed in the lumen. These cells are further hypertrophied and their cytoplasm is eosinophilic. There is an increase in the thecal vascularity.
Stage 3:
In the third stage, the lumen of the postovulatory follicle is further reduced due to continued shrinkage. There is no vascularization of the granulose cells but they contain a few blood cells. The thecal gland cells become vacuolated.
Stage 4:
The hypertrophied granulose lutein cells form a mass and occupy most of the lumen, which is drastically reduced. The postovulatory follicle becomes a multilayered structure and contains several blood cells. There is an increase in the pycnosis of ganulosa cell nuclei.
Stage 5:
In this stage the postovulatory follicle is greatly reduced. The ganulosa cells are randomly arranged and separated from each other suggesting dissolution of intercellular cohesion between them. All the nuclei are pycnotic. The thecal vascularity is deceased and it becomes more fibrous.
Stage 6:
The postovulatory follicle in this stage is reduced to a very small structure. The number of granulose lutein cells is decreased due to degeneration. The thecal layer invades the residual luteal cells. The thecal vscularity is greatly reduced.
Ovarian Cycles:
In teleosts with cystovarian ovaries, the oviducts are not the modified Mullerian ducts, but they are the posterior continuation of mesovarium or ovarian tunic. The ovarian cycle in all the fishes follows a more or less identical pattern of maturation starting from a transparent infantile ovary comprising of oocytes in cytoplasmic differentiation stage.
Followed by a growing ovary in which vitellogenesis is initiated and completed with gonadosomatic index (GSI) recording highest values. A rapid decline in GSI and simultaneous appearance of corpora lutea indicates spawning activity.
On the basis of histo-morphology the ovarian cycle of freshwater fishes is divisible into the following four stages:
Stage I:
Ovaries during the first stage are translucent structures with no externally visible ova. Oocytes are enveloped by follicular epithelium. A darkly staining body, the yolk nucleus of Balbiani (YNB) is visible clearly in the cytoplasm of oocytes. Preparedness for nucleolar extrusion is a terminal point of this stage of ovary.
Stage II:
Ovaries increase in size and oocytes become visible externally. Appearance of vacuoles along periphery and initiation of yolk deposition in oocytes are the characteristics of this stage. Nucleolar extrusion may even extend to early stage II of maturation of oocytes.
Stage III:
Ovaries are filled with ova and occupy maximum space in the body cavity. A vitelline membrane appears around the oocyte. Yolk deposition is completed. A clear theca now appears around the follicular epithelium.
Stage IV:
Ovaries exhibit loose and flaccid appearance in this stage. In this stage large number of corpora lutea of discharge follicles in different stages of formation occur. Atretic follicles of different stages are also readily seen.
Three critical phases occur in the ovarian cycle at which the control mechanism operate, viz.:
(i) Initiation of oogenesis,
(ii) Transition into vitellogenic stage, and
(iii) Spawning.
Female Reproductive Cycle:
In majority of teleosts, the ovaries are paired and of cystovarian type. Within each ovary oocytes at different developmental stages are lodged. Interstitial cells lying within the ovarian tissue are also present. A mature oocyte is characterized by the presence of three investing membranes namely, outer theca, middle follicular epithelium and innermost vitelline membrane.
Interstitial cells, theca, follicular epithelium and corpus luteum are the sites of steroid hormone production. Presence of corpora atratica is a common feature of the ovary of fishes.
Male Reproductive Cycle:
Majority of teleost fishes have paired testes (Fig. 2B). Each testis has an outer covering, the tunica, and is internally divided into a number of lobules possessing both somatic and germ cells. Various types of germ cells present within the seminiferous tubules are the sperm mother cells (primary or resting spermatogonia), secondary spermatogonia, spermatocytes (primary and secondary), spermatids and spermatozoa.
The somatic cells of testis are interstitial cells, cells lying on the boundary of lobules and phagocytes. All these cells have been suggested to be the possible sites of androgen production. Sperm duct of the teleosts is the posterior continuation of mesarchium. Two types of sperm ducts occur in teleost fishes. The first is a conspicuous gland lying anterior to the duct and the second type is the seminal vesicle.
Control of Gonadal Cycles:
External Control:
In the breeding season, two types of factors namely, the proximate and the ultimate factors exert influence. Proximate factors such as light, temperature and other physical factors regulate the development of reproductive organs and processes in breeding adult while ultimate factors like abundance of food and favorable growing conditions affect the survival of the young. Some of the more prominent environmental factors, which effect the reproductive cycle including the maturation and spawning, are discussed here.
Gonadal Maturation:
Light:
Day length is a major environmental variable synchronizing gonadal maturation with approximate season. Of all the environmental fluctuations, only length of day provides a reliable time marker for accuracy in animal breeding behaviour. In most of the fishes long photoperiod stimulates gonadal maturation.
Temperature:
Temperature is considered to be the most important exteroceptive factor controlling sexual cycles in temperate fishes. Winter dormancy is observed in the ovaries either in immature stage (stage I) or in completely mature (stage III) stage. This is an adaptation against acute cold winter conditions. This inhibitory stage is broken when the temperature starts to rise during the spring months of March and April.
Temperature and photoperiod acting together bring about maturation of the gonads. Both temperature and light influence reproduction. High temperature within the optimum range for a species is essential for efficient action of the photoperiod. Fish being poikilotherms, low temperature depresses metabolism when other environmental factors including light become ineffective.
Spawning:
Spawning, the most critical and sensitive phase in the gonadal cycle, is induced by sudden changes in climate like rise and fall in temperature or rain and floods. Temperature is an important variable effecting spawning activity of fishes in temperate areas. The role of light is indirect through the stimulation of photosynthetic activity of aquatic plants which results in a built up of dissolved oxygen content of water.
Increase in dissolved oxygen is considered to be an additional suitable habitat and stimulant for spawning activity in fishes. Day length and temperature fall during monsoon period offering suitable conditions for fish to spawn and survival of the offspring.
Rains and floods are responsible external factors favorable for triggering spawning in Indian major carps. Flood conditions created by premonsoon and monsoon rains act as stimuli for the onset of spawning. Stimulation of conditions of flooding by increasing pond water levels or by refilling Sun dried ponds induce spawning. It is not clearly known as to which specific factors created by rainfall and floods such as lowering of water temperature, dilution of electrolytes, increase in oxygen content and change of pH induce spawning.
Premonsoon rains in June cause the initiation of upstream movements of Indian carps towards their breeding grounds where they arrive and wait for flooding by monsoon rains. In the absence of such inundation, fishes evade spawning. It is observed that spawning does not occur in carps unless some rainwater is mixed with the pond water.
Other factors such as availability of food, DO and spawning grounds help in creating optimum conditions for spawners and juveniles. Spawning is sensitive to specific stimuli. Abrupt temperature changes in temperate fishes, and rainfall in tropical and subtropical species are the important triggers for spawning in Indian freshwater fishes.
In addition to established environmental factors, some social and ethological factors like visual contact, reaction to sounds of male and female, and the density of fishes in population also affect reproduction in fishes.
Endocrine Control:
The hormones secreted by the endocrine glands, chiefly the pituitary, control fundamental processes concerning development, reproduction and breeding cycles. Pituitary gland through the cyclic synthesis and release of gonadotrophic hormones regulates the gonadal cycles. Estrogens and androgens secreted by the gonads complete the cyclic chain of events through feedback systems.
Hypothalamic Control of Gonadal Cycle:
The hypothalamic neurosecretions at the time of breeding season release Gonadotrophic Releasing Hormones or factors (GnRH) and these factors stimulate the gonadotrophic cells in the anterior pituitary to secrete the gonadotrophic hormones. These hormones control the development and maturity of gonads (Fig. 3).
Hypophyseal Control of Gonadal Cycle:
Pituitary gland exerts control over reproduction. The oogonial stage of ovary is dependent upon gonadotropins. Gonadotrophic control comes into play during transition of gonads from cytoplasmic growth phase into vitellogenesis/ active spermatogenesis. Plasma vitellogenin levels and ovarian weight decrease after treatment with antiserum raised against a catfish gonadotropin fraction.
Spawning is also under the influence of gonadotropins. Heavy accumulation of secretory granules in gonadotrophs and their subsequent release shows that Leutenizing Hormone (LH) is involved during this phase. Human Chorionic Gonadotropin (HCG) also induces spawning in carps.
Gonadal Steroids:
Gonadal steroids by their action on gonads bring about the differentiation of gonoducts and gametogenesis. Estrogens exert a negative feedback control over pituitary gonadotropins during vitellogenesis resulting in an increased hepato-somatic index. Best known action of estrogens during vitellogenesis is to induce hepatic synthesis of vitellogenin, which is taken up by oocytes and the process is a gonadotropin dependent event.
Comparing the actions of estradiol, estrone and estriol in inducing vitellogenesis, estradiol is the most active steroid. In addition to estrogen, the androgen, progestin and corticosteroids are known to affect the female reproductive cycle. These steroids play a role in oocyte maturation and ovulation (Fig. 4).
Prostaglandins are also involved in induction of ovulation in fishes probably by stimulating follicular contraction. The two compounds clomiphene (antiestrogen) and cyclofenil (non-steroidal compound with hormonal properties) have different modes of action.
Clomiphene acts through hypothalamo-hypophyseal-ovarian axis while cyclofenil has a bimodal action. It acts like clomiphene and exerts effect on ovary by increasing the sensitivity of ovaries to available gonadotropins.
Essay # 3. Reproductive Cycles in Birds:
Male Reproductive Cycles in Birds:
Birds have well developed reproductive organs. In male birds, a pair of testes is present, but the females possess only one functional ovary. The gonads of birds have a dual function, secretion of hormones and production of germ cells.
Testes:
Testes occupy a dorsal position in the abdominal cavity in very close proximity of the kidney (Fig. 11). Each testis is covered by a membrane called tunica albuginia. Tunica albuginia encloses a large number of fine and coiled seminiferous tubules. Each tubule is surrounded by a membrane called tunica propria.
The inner surface of the tunica propria is lined by primary spermatogonia, which divide mitotically to form secondary spermatogonia during active phases of breeding cycle. Primary spermatocytes are formed by mitotic divisions of secondary spermatogonia. The primary spermatocytes undergo meiotic divisions to form secondary spermatocytes and spermatids. Spermatids are transformed into spermatzoa by the process of spermiogenesis.
Mature spermatozoa are present in bunches in the lumen of the seminiferous tubules during the breeding phase. Interstitial spaces are concentrated in inter-tubular spaces of inactive testis. However, interstitial cells are scattered in inter tubular spaces during progressive and breeding phases. The number of interstitial cells increases with the activity of the testis. The left testis is bigger than the right testis.
Female Reproductive Cycle in Birds:
Ovary:
In birds only the left ovary is functional. Ovary is whitish in color and irregular in shape. The size of the ovary varies from species to species (Fig. 12). Ovary is covered by a layer of germinal epithelium, which gives rise to primary and secondary follicles. The size and weight of the ovary change with the phase of the breeding cycle. The medullary part of the ovary is made up of highly vascularized connective tissue network.
Gonadal Cycles:
Birds differ in their breeding seasons. Even those living together in a particular area and sharing a common habitat breed during different seasons. The cycle of gonads can be differentiated into four phases namely, quiescent, progressive, breeding and regressive phases. The duration of each phase, the pattern of development and regression of gonads differs in different species.
Weight of the testes and size are minimum during the quiscent phase when the seminiferous tubules are of minimum diameter and contain 1 – 3 layers of spermatogonia only. Interstitial cells are present in the inter tubular spaces in small groups and do not show any secretory activity. The quiscent phase is followed by progressive phase during which, the testicular activity increases gradually.
The volume and weight also increase. Seminiferous tubules become wider and spermatogonia start to divide mitotically. In the later stages of the progressive phase, spermatocytes and spermatids are formed from the divisions of spermatogonia. Initiation of spermiogenesis indicates the beginning of the breeding phase and marks the end of progressive phase.
During this phase, due to continuous cell divisions, the germinal cells increase in population. The seminiferous tubules become stretched and their lumen contains bunches of spermatozoa.
Patterns of Gonadal Cycles:
Due to seasonal variation in climatic conditions, birds show a wide spectrum of patterns of gonadal cycles. Weaverbirds, owls, crows, buntings, munia, etc. have a sharp and short breeding phase. Other birds like parakeets and spotted munia breed over an extended period of the year. Doves and pigeons have two breeding cycles in a year.
The rate at which gonads develop during the progressive phase shows variation between different species. Regression of the gonads may be slow or fast, depending upon the Species. Photosensitive and photoperiodic birds reveal very fast gonadal development and regression. Gonadal cycles are “mainly controlled by the genetic factors and secondarily by environmental factors. Climatic factors act through the neuroendocrine system.
Body Weight Cycles:
The body weight and gonadal cycles run parallel to each other. The relationship between body weight cycles and thyroid cycles vary from species to species. Changes in the body weight are mainly due to the fat content. Increase in lipid content helps the birds to meet the increased energy requirement for successful breeding and related activities in the breeding season.
Control of Gonadal Cycles:
Birds breed at a time of the year when the environmental conditions are the most favorable for both the parents and offspring. They select such time period when the biometeriological conditions ensure the maximum chances of survival of the young ones. Thus, the evolution of seasonal breeding due to annual gonad development cycle is of great adaptive significance.
Birds have developed mechanisms to synchronize their annual gonadal cycle and breeding with the periodicity of the most favorable months or season of the year. The mechanisms, which regulate the gonadal cycle are so efficient that, they enable a particular bird species to breed regularly and periodically during the particular month or season.
Environmental Factors:
The annual gonadal cycles are conditioned by internal rhythms of reproduction. Environmental factors also exert their influence. Environmental factors include day length and monsoon. Monsoon leads to drastic changes in humidity and rainfall, landscape, quantity and quality of food, day length and intensity of light. Indirectly it provides material for building nests.
Photoperiod:
Very sensitive mechanisms involving both internal and external factors control the reproductive cycles. Some birds dependent on photoperiod can detect small variations in day length and use them as cues for regulation of gonadal cycles. In general long photoperiods have a stimulatory effect on gonadal development while short day lengths inhibit or totally abolish cycles of the gonads.
The photoperiodic response of birds differs from species to species. Birds sensitive to photoperiods become refractive at the end of the breeding phase or when exposed to long photoperiods for longer periods of time. Decreasing day length induces gonadal regression in weaverbirds. Induction of gonadal regression due to photorefractoriness can be programmed by increasing the day lengths before or during the breeding phase, or by some hormones.
Termination of the photorefractory period is mediated by inborn circadian system of measurement of the duration of photoperiods. Changes in the activity of gonads during different months and under different photoperiods are due to the alterations in the levels of leutenizing hormone releasing hormone (LHRH) and leutenizing hormone (LH) in blood and pituitary gland.
Breeding cycles of birds not influenced by photoperiods (non- photoperiodic) are under the control of hormones secreted by the thyroid, adrenal, pineal and gonads. In finches, gonadal cycles are controlled by thyroid activity. The cycles of thyroid activity and that of gonads run parallel to each other or antiparallel in some birds.
In birds with inverse thyroid – gonadal relationship, removal of the thyroid leads to development of gonads in non-breeding season, their growth and maturity and breeding. In juveniles removal of the thyroid leads to precocious development of the gonads. On the other hand, injection of thyroid hormone leads to suppression of gonadal development and maturity, and abolition of gonadal cycles.
In birds where gonadal and thyroid cycles run parallel to each other, thyroid hormone is necessary for the normal development of gonads. In such birds, removal of the thyroid glands and administration of thyroid hormone produces effects depending upon the season or month of the year. Fluctuation in the level of the thyroid hormone also has a profound effect on birds sensitive to photoperiods.
Among the internal factors, thyroid, adrenal, pineal and gonadal hormones are involved in the regulation of gonadal cycles of birds. Hormones of the endocrine glands, acting alone or in combinations and depending on the species and phases of the reproductive cycles stimulate, inhibit or totally abolish the annual gonadal cycles. These hormones change the responses of birds to photoperiods. Hormones of gonads, thyroid and adrenal are involved in the initiation and termination of photorefractoriness in birds.
Thyroid Hormones:
Annual rhythm of thyroid activity runs inversely with the gonadal cycles in a number of birds. Gradual decrease in thyroid activity in finches is followed by the gonadal development, and the increasing levels of thyroid hormones induce regression of the fully developed gonads. An inverse relationship between thyroid and gonads is found in a number of birds like jungle bush quail, spotted munia, and female weaverbirds.
Gonadal Steroids:
Testosterone and estradiol secreted by the gonads also influence gonadal cycles. However, their effect is dependent upon the dose, time of administration, species and the nature of thyroid gonadal relationship. Low doses of testosterone have an inhibitory effect on the testicular cycle while high doses stimulate the testes.
In weaverbirds, testosterone irrespective of its dose, inhibits the gonadal cycle. Gonadal steroids stimulate or inhibit the gonads acting through the hypothalamo- hypophyseal axis. However, high doses of the hormones act directly on the gonads. In temperate birds gonadal steroids play a major role in the regulation of breeding cycles. Testosterone and estradiol regulate the photorefractoriness in male and female birds.
Adrenal Hormones:
Annual activity cycles of adrenal and gonads demonstrated two types of adrenocortical-gonadal relationship namely, parallel and anti parallel type. In parallel type of relationship, exhibited by weaverbird and common myna, increase and decrease in gonadal activity is associated with respective increase or decrease in the adrenocortical tissue activity. Corticosterone exerts positive or negative effects on gonads depending on the species and phase of administration. Adrenal medullary hormone inhibits gonadal activity.
Changes in the levels of thyroid and gonadal hormones and photoperiods alter the adrenocortical activity. However, the mechanism by which thyroid, gonadal hormones and photoperiod affect the adrenocortical activity is not understood.
Pineal gland hormone also influences gonadal activity. Removal of the pineal gland stimulates gonadal activity in weaverbird even under short day length condition. Injection of melatonin inhibits the testicular cycle in weaverbirds and common myna.
Removal of the pineal gland and administration of melatonin act on the gonads by changing the levels of leutenizing hormone releasing hormone from the hypothalamus and synthesis of leutenizing hormone and follicle stimulating hormone by the pituitary gland.
Gonadal development cycle in birds thus is determined by the cyclic release of gonadotropin releasing hormones from the hypothalamus and gonadotrophic hormones by the pituitary gland. External factors like rainfall, day length and temperature influence the cyclic release of hypothalamic releasing hormones and pituitary gonadotropins.
Role of Hypothalamo-Hypophyseal Axis:
Hypothalamo-hypophyseal complex plays an important role in the regulation of reproductive cycles in birds. LH and FSH are essential for the normal gonadal development cycles in birds. In juveniles as well as in adults, gonadal activity has been reported to increase much later than the regeneration of LH-dependent pigmental plumage in male lal munia and male weaverbird.
Secretion of LH starts in the beginning of the progressive phase, reaches its peak during the breeding phase and then declines rapidly during the regressive phase to become minimum during the quiscent phase. FSH is secreted mainly during the breeding phase and stimulates abrupt testicular and ovarian growth due to induction of spermatogenesis in male and follicular growth in females.
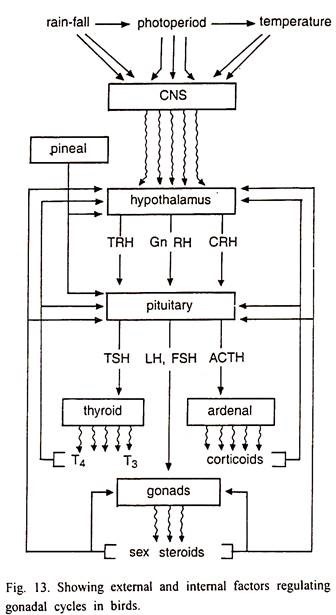
The hypothalamo-hypophyseal complex mediates effects of environmental factors and hormones on avian gonadal cycle (Fig. 13). This is the main seat of regulatory mechanisms, which regulate the circannual rhythm of gonad development in birds.
Essay # 4. Reproductive Cycles of Lizards
Male Reproductive Cycle of Lizard:
Testes:
In lizards, the male reproductive system consists of a pair of oval to round testes and epididymis, which continue as vasa deferentia on both sides and open into the cloaca (Fig. 9). Development of the germ cells takes place in the seminiferous tubules.
In active testes, the seminiferous epithelium shows spermatogenesis from spermatogonia to spermatids and spermatozoa. Leydig cells along with blood vessels, stroma elements and connective tissue fibers occupy the interstitial space between the seminiferous tubules.
Testicular Cycles:
The testes of reptiles show seasonal cycles both in the spermatogenetic activity and in Leydig cell function. Seasonal spermatogenetic cycle may or may not be synchronous with the activity of Leydig cells. Spermatogenesis in reptiles is of two types, pre-nuptial and post-nuptial. In the pre-nuptial type, sperms are produced immediately before or during mating season but in post-nuptial type, spermatogenesis takes place after the mating season.
Accordingly, in the pre-nuptial type mating occurs when the testes are spermatogenetically inactive but the sperms stored in the epididymis from the previous cycle are used during copulation. In species showing the postnuptial type, since viable sperms are stored for long periods in the epididymis during the regression of the testes, androgen production from the Leydig cells is temporarily separated from the spermatogenetic cycle. Snakes show both the types of cycles. Tropical lizards exhibiting seasonal reproductive activity are pre-nuptial and some species are reported to show continuous spermatogenesis.
The male reproductive cycle of lizards, on the basis of the size of testes, weight, histological appearance of spermatogenetic activity and the accessory reproductive organs, has been divided into three phases, the regenerative phase, reproductive phase and regressive phase. The phase of regeneration is marked by the onset of spermatogenetic activity when progressive cell divisions from spermatogonia upto spermatids occur.
This leads to the enlargement of the seminiferous tubules. The later phase shows increased size of testes and Leydig cells are few in number. Testes are biggest in size in the peak period of reproductive phase. The seminiferous tubules are maximally enlarged and the seminiferous epithelium is filled with various cell types, predominantly spermatids and spermatozoa lining the inner border of the epithelium and the lumen.
The regressive phase is marked by decrease in the size and weight of testes. The population of germ cells in the seminiferous tubules gets depleted as a consequence of which seminiferous tubules shrink. Due to tubular shrinkage, the interstitium and the Leydig cells become conspicuous. The duration of the regressive phase varies with the type of cycle. In lizards, the regressive period is generally longer extending upto six months after mating.
In testes, Leydig cells show well-defined seasonal lipid cycles. A slow postnuptial accumulation of cholesterol positive lipids in the Leydig cells takes place during the regressive phase. In the testes of snakes, the atrophic lipoidal Leydig cells are replaced by a new generation of Leydig cells.
Cycle of Seminiferous Tubules:
In seasonal breeders, the seminiferous tubular epithelium shows conspicuous changes such as the postnuptial accumulation of lipids, which are cleared with the onset of testicular recrudescence. Sertoli cells show cyclic lipid accumulation and depletion along with the spermatogenetic cycle.
In the lizards accumulation of cholesterol positive lipid material follows the spermatogenetic cessation after the breeding season. The seasonal lipid cycles in the testes are related to the seasonal spermatogenesis and secretion of gonadotropins from pituitary gland.
Cycle of Lipid and Cholesterol:
In the annual reproductive cycles of reptiles, quantitative changes occur in the lipid, cholesterol and other steroid levels of the testes. In lizard testes, the lipid content is highest in the regressed phase and dramatic reduction occurs during the spermatogenetic recrudescence and lowest during the active phase. With the pos nuptial testicular regression, the lipid content again increases.
Cholesterol level also shows seasonal changes during the reproductive cycle. It is highest during the post nuptial lipid accumulation period and decreases with the initiation of spermatogenetic recrudescence suggesting active mobilization possibly by steroidogenesis. In some lizards cholesterol esters show sharp increase in the post breeding period and decrease during the recrudescence of the spermatogenesis.
Female Reproductive Cycles of Lizards:
Ovary:
In lizards ovaries are bilobed, irregular and small structures lying in the body cavity attached to the dorsal body wall by means of mesenteries (Fig. 10). A limiting membrane consisting of a layer of squamous epithelium restricted to the dorsal surface surrounds each ovary.
The oogonia and oocytes are located in discrete areas known as germinal beds. The number and distribution of the germinal beds varies in different species. In some lizards and snakes there are two germinal beds in each ovary lying one on either side of the ovary.
Oogonia are smaller in size as compared to oocytes and contain little cytoplasm with a small centrally located nucleus. The primary oocytes are larger in size and contain more cytoplasm. Their nuclei are centrally placed, larger in size and vesicular in shape. The nucleolus stains deeply.
A single layer of granulosa cells surrounds the primary oocytes. As the primary oocytes grow in size, the granulosa cells are pushed out of the germinal bed. At this stage they are called as the developing follicles. The zona pellucida is surrounded by a 3-4-ceIl thick granulosa layer.
The cells in the granulosa layer are polymorphic consisting of three types of cells, small, intermediate and large. A layer of connective tissue called as theca surrounds the granulosa externally. The theca is further differentiated into an outer theca externa and an inner vascular theca interna.
In lizards the ovarian cycle runs parallel to the testicular cycle. The seasonal changes in the development of the ovary are complex and can be differentiated into five stages. These include periods of regeneration, vitellogenesis, ovulation, gestation and regression.
During the period of regression, the ovaries are inactive and dormant showing no signs of growth. Decrease in weight follows the degeneration of ovarian components like corpora lutea and the follicles, which did not undergo vitellogenesis.
Towards the end of the regression period, some lizards like Mabuya carinata show slight increase in the weight of their ovaries. Ovarian follicles show signs of development and few follicles are in the stage of vitellogenesis.
In the regeneration phase there is a gradual increase in the weight of ovaries and conspicuous proliferation of the germinal beds with several oogonia and primary oocytes at different stages of development. A large number of developing follicles, a few persistent corpora lutea and atretic follicles of the previous cycles show various stages of degeneration.
Vitellogenesi stage is characterized by the process of yolk formation and deposition in the previtellogenesis follicles. Vitellogenesis continues for 30 – 40 days. In the beginning of the vitellogenesis stage, yolk accumulates at the periphery of the cortical ooplasm of the follicles.
In the period of ovulation the ovaries are heavy due to the presence of mature yolk follicles. Ovulation is preceded by a break in follicular epithelium. All the mature ova are expelled simultaneously from both the ovaries into the abdominal cavity from where they migrate to the oviducts. Transovarian migration of ovulated eggs is very common and this can be identified by the difference in the number of eggs in the oviduct and the number of corpora lutea in each ovary.
Mating occurs just before or at the time ovulation. Fertilization takes place in the infundibulum of the oviduct and the fertilized eggs are coated with albumen, shell membrane and shell in the oviduct. Eggs are retained in the uterus for different periods of time before oviposition. After ovulation, the follicles develop into corpora lutea and retain their activity for a part or full length of the period of gestation.
Control of the Gonadal Cycles:
Endocrine Control:
Pituitary gonadotrophs show cyclic changes in their secretory activity during seasonal reproductive cycles of reptiles. The role of pituitary hormones in controlling the testicular and ovarian cycles is an established phenomenon in reptiles.
Thyroid hormones exert both positive and negative effects on the gonads of reptiles. Removal of the thyroid glands results in regression of the gonads and this effect can be reversed by injection of thyroxin. Administration of thyroxin in the active phase produces antispermatogenetic effects.
Environmental Control:
In reptiles, reproductive cycles are influenced by rainfall, relative humidity, temperature and length of the day. Direct influence of rainfall on reproduction is not established in temperate reptiles. Indirectly rainfall can change the temperature of the microclimate and food availability. In tropical reptiles, rainfall influences reproduction in females. Egg laying is dependent on moisture content.
Photoperiod initiates the seasonal recrudescence of the testes. Photo-receptiveness is restricted only to a short period in the annual cycle and at other times of the year lizards are not receptive to photoperiod. Effects of photoperiod are temperature dependent. Low temperature is inhibitory while high temperature is stimulatory.
Long photoperiods with high temperature stimulate testicular recrudescence in autumn. Temperature plays a primary regulatory role in the reproductive cycles of reptiles as compared to rainfall or photoperiod. In many lizards and snakes, increasing temperature stimulates ovarian development and testicular recrudescence.
Essay # 5. Reproductive Cycles of Frogs:
Male Reproductive Cycle of Frog:
Testes:
Testes in anurans are paired; ovoid and compact structures lying near the kidneys. A short mesarchium connects them dorsally to the body wall. The vasa efferentia connecting the seminiferous tubules and the modified nephric elements running to the Wolffian ducts also travel through the mesarchium.
The germ cells are endodermal in origin. The two main elements of the testes are the seminiferous tubules and the interstitial tissue consisting of connective tissue, blood capillaries and closely packed small ovoid steroid secreting Leydig cells. The seminiferous tubules obtain their nutrients and other substances chiefly by diffusion from the vascular interstitial areas.
Spermatogenesis:
Spermatogenesis in amphibians is cystic type. Cells present in the cyst are derived from a single spermatogonium and are all in the same stage of development. However, a cross section of the seminiferous tubule shows cysts in different stages of spermatogenesis (Fig. 5).
The different stages of spermatogenesis are as follows:
Stage O:
Primary spermatogonia.
Stage I:
Secondary spermatogonia.
Stage II:
Primary spermatocytes.
Stage III:
Secondary spermatocytes.
Stage IV:
Spermatids.
Stage V:
Sperm bundles attached to the Sertoli cells.
The primary spermatogonia are the largest sperm cell type present in the adult testis. Each consists of large amount of eosinophilic cytoplasm and irregularly shaped nucleus. They lie singly adjacent to the basement membrane of the seminiferous tubule, each with one or two supporting cells associated with them.
Primary spermatogonia divide mitotically into two daughter primary spermatogonia or form a cyst through repeated mitotic divisions. The cyst is composed of the secondary spermatogonia, which divide repeatedly constituting the proliferation or multiplication phase.
About 8 divisions occur between the beginning and end of the multiplication phase. At the end of the last multiplication division, both cytoplasm and nucleus of the cells increase in size, and the cytoplasm becomes eosinophilic.
At the end of this growth period, the cells become somewhat larger in size, the cytoplasm entirely eosinophilic and the basophilic nuclei enter into the pre-reduction stage. These cells are now known as the primary spermatocytes. They lie either in the central part of the testis or attached to the wall of the seminiferous tubules.
The primary spermatocytic cysts show various stages of meiotic divisions. The first meiotic division produces secondary spermatocytes characterized by eosinophilic cytoplasm and strongly basophilic nuclear chromatin. The intercellular vacuoles increase in size and become more evenly distributed. The cell nests are located in the central parts of the testis tubules.
After the second meiotic division, spermatids are formed. The spermatids are smaller than the secondary spermatocytes and are globular cells, with eosinophilic cytoplasm and a spherical basophilic nucleus. With the starting of the spermatogenesis they become more and more elongated.
The intercellular vacuoles fuse into one big central vacuole and the cells are situated against the wall of the cyst. This type of cell nests is usually found attached to the wall of the seminiferous tubules. The heads of the maturing sperm cells are found embedded in the Sertoli cells. Each cell nest changes into about a dozen sperm bundles.
Sertoli Cells:
Sertoli cells are the only somatic elements found inside the seminiferous tubules. The Sertoli cells initially associated with the primary spermatogonia are flattened or triangular in shape with small, oval nuclei. The chromatin material is condensed. There may be 2-4 such cells surrounding a primary spermatogonium.
These are the follicular cells with the progress in spermatogenesis and formation of the cell nests of advanced stages; the Sertoli cells undergo progressive differentiation. The heads of several spermatozoa are found embedded in such fully differentiated cup-like Sertoli cells. They are generally attached to the basement membrane of the tubule wall and after spermiation, the cells burst and they are then visible freely floating in the lumen of the tubule.
Leydig Cells:
The interstitial cells or Leydig cells are small, compact and oval cells distributed between the seminiferous tubules. The size and shape of these cells as well as the morphological features of their nuclei show seasonal changes depending upon the species.
The Leydig cells, spherical in shape with large nuclei, conspicuous nucleoli and coarse chromatin granules are considered to be actively secreting androgens. Flat Leydig cells with reduced size of nuclei and possessing compact chromatin are inactive.
Testicular Cycles:
Spermatogenetic Cycles:
In frogs, production of primary and secondary spermatocytes, spermatids and sperm bundles occurs round the year showing that maturation of germ cells is not restricted to any specified part of the year. There is no appreciable seasonal variation in the rates of mitotic or meiotic activities.
The maximum weight attained by the testes during the breeding season is only 1.5 times of that found in other months. This is because proliferation and maturation of germ cells occur simultaneously and more or less at uniform rate through out the year. Secondary spermatogonia are produced through out the year but the mitotic activity in them is very prominent between April to June.
The primary and secondary spermatocytes, spermatids and sperm bundles are rapidly produced between April to June. It is interesting to note that even though secondary spermatogonia continue to divide and give rise to primary spermatocytes during the post-breeding period, very few secondary spermatocytes are formed in September – October 2nd these eventually undergo degeneration during November to February (Fig. 6).
In anurans living in the temperate zone, increase in the weight of testis —arks the proliferation of new germ cells and later with the onset differentiation or maturation of germ cells there is a decrease in the weight of testes. However, in the tropical anuran, both proliferation and maturation of germ cells occurs concurrently, the testis weight declines only after the evacuation of the spermatozoa during the breeding season.
Cycles of Leydig Cells and Steroidogenic Activity:
Leydig cells are round, abundant and exhibit maximum nuclear diameter during the breeding period. The nuclei of Leydig cells contain coarse chromatin granules in the of May and June. After breeding, the Leydig cells become flattened of shrunken and reduced in size and number.
They become less frequent but distinguishable from the connective tissue cells though with some difficultly. In this period they contain fine chromatin granules. Leydig cells continue to remain in this stage from August to March. In the months of April and May they rapidly increase in size and number, and become round.
The redistribution of chromatin material takes place due to which the coarse chromatin granules characteristic of secondary cells appear once again appear in their nuclei. In toads Leydig cells are round, abundant and show maximum nuclear diameter in April to August. This is followed by a marked decrease in nuclear diameter of Leydig cells during August – September.
However, from September to March, no further changes appear in the morphology of these cells. In April, Leydig cells number, size and chromatin granules content increase upto August. Thus, no drastic changes are noted in the Leydig cells of toads.
Control of Testicular Cycles:
The hormonal and environmental factors control and effect the annual changes in the testes. Temperate amphibians due to the lack of thermoregulatory mechanisms are greatly influenced by the changes in the temperature. Spermatogenesis does not take place during cold winter months and therefore, this constitutes the resting period.
Spermatogenetic activity is very high in summer months. Thus they show potentially continuous or discontinuous type of spermatogenetic cycles. Testicular cycles are controlled by both intrinsic and extrinsic factors.
Pituitary Control:
Gonadotrophic hormones produced by pituitary gland play important role in the regulation of spermatogenesis in all the vertebrates. In adult amphibians, mitotic proliferation of primary and secondary spermatogonia is dependent upon gonadotrophic hormones.
Annual changes in the testes are due to the influence of gonadotropins on the germinal epithelium. In green frog, the sensitivity of the germinal epithelium and the gonadotropin levels of the pituitary gland were higher in summer season. Even after the breeding season, testis of frog retains its sensitivity to gonadotropins.
In anuran, the activity of Leydig cells is also regulated by the hypophysial gonadotropins. Removal of the pituitary gland causes regression of these cells, which can be prevented by pituitary homogenates.
Role of Androgens:
The main sources of androgens in anuran are the Leydig cells. Androgens stimulate specific stages of spermatogenesis, development and maintenance of the secondary sexual characters such as the thumb pads and vocal sacs, and feedback regulation of pituitary gonadotropin secretion. The development and regression of thumb pads is due to the fluctuations in the leaves of androgens secreted by the Leydig cells.
In anurans of temperate zone, the annual cycles of Leydig cells activity is not the same as spermatogenetic cycle. Thumb pad development and Leydig cell activity are high when spermatogenetic activity is nil, but during the period of recovery of spermatogenetic activity, the thumb pads atrophy and the Leydig cells become inactive.
Environmental Factors:
High temperatures between 20 to 25° C stimulate spermatogenesis but suppress the activity of Leydig cells in anurans. In winter low temperature has the opposite effect. In tropical countries rainfall is an important factor controlling the breeding activity as the frogs breed during the monsoon months.
Female Reproductive Cycle of Frog:
Ovary:
Ovaries of frogs are paired structures attached to the median surface of the kidneys by mesovarium. Each ovary is a hollow sac-like structure. Its wall is thrown into numerous folds and consists of a narrow cortical region covered by germinal epithelium.
Depending upon the phase of reproductive cycle, or the pattern of oogenetic activity, the ovary may contain oogonia, previtellogenic oocytes of different sizes, vitellogenic and fully-grown yolky oocytes. The corpora lutea are found in the ovaries during the immediate post-spawning period (Fig. 7).
Oogenesis:
Oogenesis starts even in larvae before their metamorphosis. At the time of metamorphosis, the larva contains oogonia and primary oocytes that have entered their first growth phase of early diplotene stage. The oogonia possess large and highly lobed nuclei. Each nucleus contains a large, prominent nucleolus and many micronucleoli.
The oogonia are found scattered in the peripheral cortex of the ovary. The oogonia persist in the ovary of adults and are therefore found at all times in the germinal epithelium. The appearance of yolk vesicles marks the beginning of the vitellogen or the second growth phase of the oocytes.
Follicular Atresia:
Atretic follicles are commonly found in the ovary of amphibians and the histological changes that occur during atrophy of the follicles are divided into four stages:
Stage 1:
The characteristic features of the atretic follicle at this stage are hypertrophy of the follicular granulosa cells, appearance of vacuoles at the periphery of the oocyte, and coalescence of yolk platelets at the periphery of the oocyte.
Stage 2:
In this stage, the zona pellucida and follicular epithelium are not visible. The hypertrophied granulosa cells move into the ooplasm or the yolk material of the egg, forming conspicuous epithelial cells, and possess clear, vacuolated cytoplasm and a vesicular nucleus. The theca becomes vascular.
Stage 3:
The ooplasmic contents of the large, previtellogenic follicles are digested and removed by the phagocytic granulosa cells, leaving behind the hypertrophied thecal cells in the connective tissue.
Stage 4:
There is a gradual degeneration and disappearance of the phagocytic granulosa cells, leaving only the pigment and the original elements of the theca.
Corpus Luteum:
The follicular membranes remain behind in the ovary after ovulation and give rise to post-ovulatory follicles or corpora lutea.
The histological changes occurring in the corpora lutea can be divided into the following four stages:
Stage 1:
This stage is characterized by the newly ruptured follicle, the follicular epithelium of which is almost similar to that of the preovulatory follicles. The thecal wall contains capillaries with blood and the tissue is vacuolated. The inner layer is formed of a dense continuous membrane.
Stage 2:
Both granulosa and thecal cells are relatively more hypertrophied. The follicle becomes collapsed and folded due to contraction.
Stage 3:
In the third stage the granulosa becomes multilayered due to further shrinkage or contraction of the follicle and the cavity of the post-ovulatory follicle is almost filled up by the hypertrophied granulosa cells. The hypertrophy of the theca is maximum at this stage.
Stage 4:
Further contraction of the follicle occurs at this stage and the granulosa cells are separated from each other and are irregularly distributed. There is a further reduction in size.
Stroma and Interstitial Cells:
The stroma of the ovary of frogs consists of cellular and fibrous connective tissue elements, which is distributed between the follicles, corpora lutea, interstitial gland cells, blood vessels, lymphatic spaces and nerve endings. The quantity and distribution of the ovarian stroma undergo marked seasonal variation in response to growth and recruitment of follicles. The interstitial gland cells are very scarce in the anuran ovary.
Ovarian Cycles:
The Morphology of the Ovary in Amphibians:
The morphology of the ovary of anurans varies with the phase of reproductive cycle and seasonal changes in the recruitment of oocytes, atresia and ovulation. During winter months due to the drastic decrease in temperature, development of the follicles is interrupted.
This inactive resting phase varies from species to species and ranges between 1 to 4 months. Oogenesis mainly occurs during spring and summer months. Vitellogenesis is completed either just prior to breeding or immediately after breeding.
In some frogs like Rana cyanopeltis oogenesis occurs throughout the year due to continuous oogenetic activity. About 25% of the total oocytes complete the vitellogenic growth and reach ovulatory sizes in May -June each year.
Breeding season spreads over 2-3 months from July to September and a mature female may shed as many as 300 eggs. Proliferation of oogonia and recruitment of oocytes start soon after the breeding months. Weight of the ovary is dependent upon the number of SGP oocytes.
Although atretic oocytes occur in all the months of the year, their number is higher during pre-breeding and breeding months. Toads breed during the monsoon months of June to August. In temperate anurans, the ovaries enter a period of quiscence before the initiation of the next ovarian cycle by recruiting a batch of small oocytes to the final growth phase.
Cycles of the Oviduct and Fat Bodies:
Seasonal changes also occur in the oviducts in correlation with the changes in ovaries. Seasonal variation in the weight of the oviducts is related to the number of SGP oocytes. Abdominal fat bodies also show seasonal changes in size and weight. Cycles of the fat bodies are inversely correlated to the ovarian cycles except during the immediate post-spawning period during which both the ovaries and fat bodies are reduced.
The reproductive cycles of amphibians are influenced by the changes in climatic factors such as temperature, rainfall, day length and relative humidity as they are poikilotherms and their breeding activities require water. Temperate amphibians show distinct seasonal changes in the ovarian follicular development, period of breeding and hibernation but anurans in tropical areas do not show such sharply defined seasonal changes in the gametogenetic activity.
Ovarian cycle in all amphibians is regulated by both intrinsic and extrinsic factors. The intrinsic factors mainly include the hypophyseal gonadotropins and ovarian estrogens. The extrinsic factors are temperature, light, rainfall and relative humidity. Sufficient food supply and water are essential.
Endocrine Control:
For normal functioning of the ovaries, hypophyseal gonadotropins are necessary. Removal of the pituitary gland or injection of antigonadotrophic chemicals causes degeneration of the ovary. However, the individual role of follicle stimulating hormone (FSH) and LH are less effective in amphibians because of their short half-life (Fig. 8).