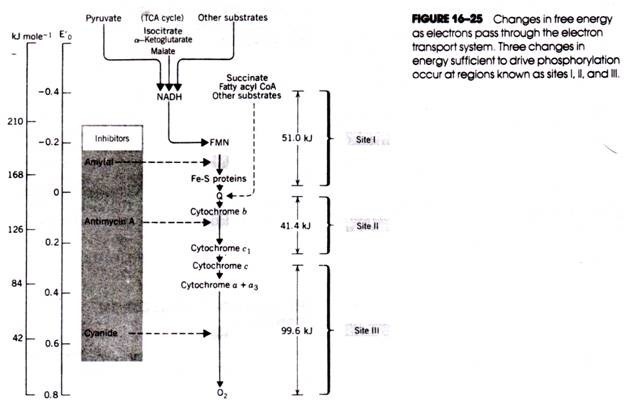
Here is an essay on the ‘Life Cycle of Gnetum’. Find paragraphs, long and short essays on the ‘Life Cycle of Gnetum’ especially written for school and college students.
Essay # 1. Sporophyte of Gnetum:
Gnetum resembles very much in its characteristics to an angiosperm than a gymnosperm. Based on the studies of 58 characteristics, Gnetum shares more than 60% of the characteristics with angiosperm and about 30% characteristics with gymnosperm. Gnetum is easily mistaken for a dicot plant unless it is in flowering or fruiting stage. The plant body is differentiated into root, stem and leaves.
1. Root of Gnetum:
External Morphology:
Gnetum shows a typical tap root system which is profusely branched. The mature roots show normal secondary growth.
Internal Structure:
Internally, the root of Gnetum resembles the root of angiosperm. The root is differentiated into the outermost layer, epidermis, multilayered cortex and diarch vascular cylinder. The cortex consists of polygonal or oval-shaped parenchymatous cells containing starch grains. The thick-walled fibre cells are often present in the cortex. A single-layered endodermis encircles a multilayered pericycle.
The primary vascular cylinder is diarch, radial and exarch. The secondary growth in roots is of normal type. The arcs of cambium form below the phloem groups and above the xylem groups which join together to form a cambium ring. The secondary xylem consists of tracheids possessing uniseriate bordered pits with conspicuous Bars of Sanio. The vessels are also present.
The pits on the vessels are bordered or simple, small and multiseriate with or without inconspicuous Bars of Sanio. The xylem ray is composed of thin-walled parenchymatous cells containing starch grains. The phloem consists of sieve cells and parenchyma. The periderm is formed due to the extrastelar secondary growth.
2. Stem of Gnetum:
External Morphology:
Almost all the species of Gnetum, except the tree type like G. gnemon, exhibits two types of branches viz. dwarf shoots or branches of limited growth and long shoots or branches of unlimited growth. In climbing and scandent species of Gnetum, the stem is articulated with prominent joints.
The joint consists of two parts: one just above the node and the other below the node and these two are separated by an annular groove. In arboreal species such as G. gnemon, the stem exhibits uniform type of branching.
Internal Structure:
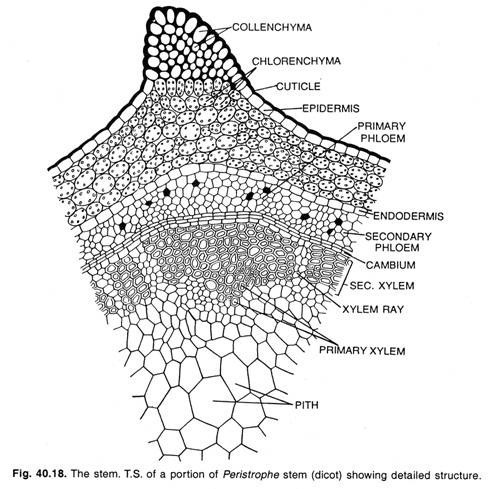
Internally, a Gnetum stem is similar to that of a dicot. In T.S. the stem exhibits more or less circular outline (Fig. 1.71 A). The following regions are discernible from outside inward: the heavily cutinised single-layered epidermis with sunken stomata, a several- layered (12-16 layers) thick cortex which is differentiated into an outer chlorenchymatous, a middle parenchymatous and an inner sclerenchymatous region.
The cells of the inner regions are referred to as spicular cells (Fig. 1.71C). Many fibrous cells are often present which have branched or unbranched pit canals. The cortex is followed by endodermis and pericycle layers which are not distinct. Next to pericycle, there is an endarch siphonostele.
The vascular cylinder is comprised of 20-24 collateral, open and endarch vascular bundles, arranged in a ring (Fig. 1.71 A). The vascular bundles are separated from one other by wide primary medullary rays. The xylem consists of tracheids and few vessels (Fig. 1.71 B).
The protoxylem of tracheids have annular or spiral thickening and the metaxylem have reticulate thickening with uniseriate bordered pits. The phloem is composed of sieve cells and phloem parechyma. Laticiferous elements are often found in pith as well as in cortex.
Secondary Growth in Thickness:
In tree species like G. gnemon, the secondary growth is of normal type. The fascicular cambium joins with interfascicular cambium to form a complete cambium ring that cuts off a continuous cylinder of secondary xylem towards the inside and secondary phloem towards the outside. The secondary xylem is produced much more than the secondary phloem.
In climbing species (G. ula, G. africanum), the anomalous secondary growth is noted. Initially, the stem shows normal secondary growth. Later, several successive cambium rings are formed in the cortex.
Thus, several extra- stellar rings of xylem and phloem are formed which are separated into wedge-shaped bundles because of medullary rays (Fig. 1.71D). Some of these accessory rings remain incomplete and, as a result, the vascular bundles become eccentric with regard to pith (Fig. 1.71 E).
The periderm is also formed by the activity of phellogen during the third or fourth year of growth of the stem.
The wood of Gnetum is composed of vessels, tracheids and xylem parenchyma. The vessels are of different sizes and have a single pore on their end walls (Fig. 1.72A). The tracheids are much longer than the vessels and have bordered pits on both of their radial and tangential walls. The xylem parenchyma cells have simple pits. There are many uni- to multiseriate vascular rays (Fig. 1.72B, C).
The secondary phloem consists of sieve cells and phloem parenchyma. The companion cells are totally absent in Gnetum, although some small cells are present near the sieve tube.
The vascular rays are very large and multi- seriate which appear boat-shaped in T.L.S. Occasionally, some short biseriate or uniseriate rays are present. The ray cells are thick-walled and pitted.
3. Leaf of Gnetum:
External Morphology:
Gnetum exhibits leaf dimorphism bearing both the foliage leaves and scale leaves.
A dwarf shoot bears 9 to 10 foliage leaves, arranged in an opposite decussate manner. The leaves are large, simple having an oval-shaped broad coriaceous lamina with unicostate reticulate venation. The leaves are exstipulate, shortly petiolate with entire margin. The leaf of Gnetum resembles a dicot leaf.
Internal Structure:
Anatomically, the leaves of Gnetum resemble those of dorsiventral dicot leaves. The epidermis (both upper and lower) has undulating walls, covered with thick cuticle. The mesophyll is differentiated into palisade and spongy parenchyma.
The palisade consists of single-layered compactly arranged elongated cells. The spongy parenchyma is composed of loosely arranged lobed cells. In addition, some spicular cells, sclerotic cells, latex tubes and fibres are also present.
The vascular bundles are arranged in a curved in the midrib region. Each bundle is of conjoint, collateral and endarch type with an adaxial xylem and abaxial phloem. There are distinct patches of stone cells outside the phloem.
The xylem is composed of vessels, tracheids and xylem parenchyma, while the phloem consists of sieve cells and phloem parenchyma. Transfusion tissue is present in the petiole in association with vascular bundles.
According to Takeda. (1913) and Florin (1931), the stomata of Gnetum is of syndetocheilic type (i.e., both the guard cells and subsidiary cells develop from a common stomatal initial). But, Maheswari and Vasil (1961) reported that the development of stomata in G. ula is haplocheilic where the stomatal initial forms only guard cells, while irregularly arranged epidermal cells around them do not function as subsidiary cell.
Essay # 2. Reproduction:
Gnetum reproduces sexually. Gnetum is dioecious and both the male and female strobili (= inflorescence) are compound. The inflorescence is either axillary or terminal in position which occurs singly or in groups. The inflorescence is composed of a stout long axis with two opposite decussate, connate bracts at the base and a series of cup-like bracts called cupules or collars that are superposed one above the other (Fig. 1.73, 1.74A).
The strobilus becomes compact at young stage because of the suppression of internodes. Thus, the collars of a young strobilus appear to be continuous (Fig. 1.73). At maturity, the axis becomes elongated and the collars get separated. There are many rings of flowers in the axil of collars. The collars are developed in acropetal succession and the flowers are initiated as mounds of meristematic cells from the lower surface of a collar.
Male Strobilus:
The male strobilus is compound and has a long slender axis bearing 10-25 whorl of bracts (collars) (Fig. 1.73). About 12-25 male flowers are arranged in three to six rings above each collar (Fig. 1.74A). A single ring of 7-15 imperfect female flowers or abortive ovules is present just above the male flowers.
Male Flower:
A male flower consists of two unilocular anthers on a stalk (antherophore) enclosed in a small sheathing perianth (Fig. 1.74B). The stalk of the anther elongates rapidly at maturity pushing the anther beyond the collars through a slit in the perianth.
Female Strobilus:
The female strobilus is very much similar to that of the male strobilus in the young stage. Like male strobilus, the female strobilus consists of an axis bearing several whorl of collars arranged one above the other (Fig. 1.75A). A ring of 4-10 ovules (female flowers) is present above each collar (Fig. 1.75B). The male flowers are not found in the female strobilus. The upper few collars are devoid of ovules and are thus sterile (Fig. 1.75B).
Ovule:
A single ovule represents a female flower. The ovule is stalked in G. ula, but may be sub- sessile or even sessile. The ovules are orthotropous, crassinucellate (with massive nucellar tissue) and are protected by three envelopes (Fig. 1.75C). The outer envelop which becomes thickened and succulent at maturity is considered to be the perianth corresponding to the perianth of male flower.
The middle and the inner envelopes are actually the integuments. Numerous laticiferous ducts and sclerides are present in the perianth with some epidermal stomata. The middle envelop is called the outer integument which is anatomically similar to the outer envelop. The inner envelop, i.e., the inner integument, elongates far beyond the apical cleft of the perianth and forms a long micropylar tube (Fig. 1.75C).
Anatomically, the inner integument is different from the other two envelopes, because neither sclerides nor stomata develop in the inner integument. The inner integument is free from the nucellus except at the chalazal end.
Two sets of vascular bundles are formed (Fig. 1.75C), of which the outer set passes to the perianth and the inner set again divides and one of its branches passes to the outer integument and the other to the inner integument. All the three envelopes of ovules develop in acropetal manner. A shallow pollen chamber is present at the tip of the nucellus.
Megasporogenesis:
Two to four hypodermal cells in the nucellar tissue at the micropylar end is differentiated into primary parietal cells towards outside and the primary sporogenous cells towards inside. The primary parietal cells together with nucellar epidermal cells divide repeatedly to produce a massive nucellus.
The primary sporogenous cells divide to form 8-20 sporogenous cells which are linearly arranged. The sporogenous cells function as megaspore mother cells which undergo meiotic division. Since no walls are laid down after meiotic division of megaspore mother cells, all the four nuclei remain within the mother cell to form a tetranucleate coenomegaspore. Thus, the female gametophyte of Gnetum is tetrasporic.
Essay # 3. Gametophyte of Gnetum:
The spore is the first phase of gametophyte generation. The microspore or pollen grain represents the male gametophyte, while the tetranucleate coenomegaspore represents the first phase of female gametophyte which develops into a female gametophyte.
Development of Male Gametophyte before Pollination:
The pollen grain is inapeturate and spherical, bounded by the two concentric wall layers: the outer thick, spiny exine and the inner thin intine (Fig. 1.74C). The pollen nucleus divides mitotically to produce a small lens-shaped prothallial cell and a large antheridiai initial (Fig. 1.76B). The prothallial cell does not divide further and eventually degenerates.
The antheridiai initial divides to form an antheridiai cell and a tube nucleus (Fig. 1.76C). The antheridiai cell directly functions as spermatogenous cell. The pollen grains are released from the microsporangium at this 3- celled stage (one prothallial cell, an antheridiai or spermatogenous cell and a tube nucleus).
According to Thomson (1961), the prothallial cell is not formed in Gnetum. He proposed that the pollen nucleus cuts off a tube nucleus and a generative cell (Fig. 1.76B’). The generative cell again divides forming a stalk cell and a body cell (Fig. 1.76C’). Thus, the pollen grains are released at this 3-celled stage (tube nucleus, stalk cell and body cell).
Development of Male Gametophyte after Pollination:
The exine is cast off during pollen germination. The tube cell of the pollen comes out in the form of a pollen tube which traverses the nucellar tissue through intercellular spaces. The prothalial cell remains within the pollen grain and eventually disorganises.
The spermatogenous cell moves into the pollen tube and subsequently it divides to form two equal (e.g., G. ula, G. gnemon) or unequal (e.g., C. africanum) male cells just prior to fertilisation (Fig. 1.76D). The male cells are actually the male gametes which are non-motile. However, according to Thomson (1961) the stalk cell remains within the microspore and eventually degenerates. The body cell moves into the tube where it divides to form two male cells (Fig. 1.76D’).
Development of Female Gametophyte:
At the initial stage, before the gametophyte formation, the nucellar cells immediately below the megaspore mother cell divide to form a tissue. The cells of this tissue are arranged in radiating rows. This tissue is termed as ‘pavement tissue’ which eventually gets absorbed and seems to be nutritive in function.
There is a free nuclear division in the coenomegaspore, as a result a large number of free nuclei are formed (Fig. 1.77A). The number of nuclei thus formed varies in different species, viz. 256 in G. gnemon, 512 in G. africanum and 1500 in G. ula. At this stage, a large central vacuole appears and the free nuclei lie in a thin film of cytoplasm around the vacuole towards the periphery (Fig. 1.77B). Later, the nuclei in the peripheral cytoplasm divide repeatedly.
At this stage, the upper part of the gametophyte surrounding a vacuole widens, while the lower part of the gametophyte shows accumulation of cytoplasm. Thus, the gametophyte becomes an inverted flask-shaped structure (Fig. 1.77C). The wall formation starts very slowly from the chalazal end towards the micropylar end. Thus, the nuclei remain free at the microphylar end even at the time of fertilisation.
The important characteristic in the female gametophyte of Gnetum is the absence of archegonia. One to three nuclei of the gametophyte in the micropylar end enlarge several times and accumulate dense cytoplasm around them. These large and densely cytoplasmic cells are the eggs (Fig. 1.77B). It is important to note that all the eggs do not mature simultaneously.
Pollination:
Gnetum is wind-pollinated. The pollen grains are dispersed from the anther and remain suspended in the air for some time. At the free nuclear stage of the female gametophyte, the nucellar beak in the ovule disorganises forming a viscous sugary liquid which comes out through the microphyle in the form of a pollination drop. The pollen grains are caught in the pollination drop.
Due to the drying off of the fluid, the pollen grains are sucked into the micropylar canal and are finally collected in the pollen chamber. The mouth of the micropyle is then sealed from the outer environment due to the development of flage (a circular rim or an umbrella-shaped structure develops from the inner integument) and micropylar closing tissue (a tissue develops by the proliferation of the inner epidermis of integument at the level of flage).
Fertilisation:
The pollen tube enters the female gametophyte and the male gametes move ahead of tube nucleus (Fig. 1.77B). The pollen tube ruptures to discharge the male gametes into the egg cell.
The cell sheath of male cell is left outside the egg cell. Usually one of the male nuclei fuses with the egg nucleus and thus a zygote is formed. Sometimes, two male gametes may fuse two different eggs if those eggs are in the vicinity of the pollen tube.
Endosperm:
In gymnosperms, endosperms are cellular and haploid and are formed before fertilisation. However, in Gnetum the development of endosperms starts before fertilisation very slowly from lower part of the gametophyte which eventually proceeds upward. After fertilisation, the wall formation starts in such a way that the cytoplasm divides into many multinucleate compartments (Fig. 1.78A). Later, the nuclei in each cell fuse to form a single polyploid nucleus (Fig. 1.78B).
In this stage, the lower part of the gametophyte becomes cellular, while the upper part remains free nuclear even after fertilisation (Fig. 1.77C). Thus, the development of endosperm takes place even after fertilisation. There is a great variation in the development of endosperm in Gnetum.
In some cases, the wall formation starts either from the upper part or from the middle part of the gametophyte instead of the lower part and the whole gametophyte may become cellular. Though some portions of the endosperms are formed after fertilisation, the characteristic triploid endosperm through double fertilisation is, however, absent in Gnetum.
Embryogeny:
In all angiosperms (except, Paeonia), the division of zygote is accompanied by wall- formation; while in all gymnosperms (except Sequoia, Welwitschia), there is a free nuclear phase in the zygote during the development of embryo. However, Gnetum occupies an intermediate stage between gymnosperms and angiosperms with regard to embryo development by having both the free-nuclear divisions as well as cell divisions.
There is a great variation in the early development of the embryo in different species of Gnetum.
In C. gnemon, the zygote develops 1-3 small tubular outgrowths. Only one of the pro-tuberances receives the nucleus and survives, while the remaining protuberances die out (Fig. 1.79A).
The surviving tubular outgrowth becomes much elongated and branched and develops in various directions invading the intercellular spaces of the endosperm. These tubes are called primary suspensor tubes or proembryonal tubes (Fig. 1.79B).
All the primary suspensor tubes remain coiled around each other. At the tip of the primary suspensor tube, a small cell is cut off which eventually divides by a transverse wall, followed by a longitudinal wall resulting into four cells.
This is further followed by irregular divisions to form a group of cells. Now, further divisions take place in some of these cells which eventually elongate to form secondary suspensor. The rest of the cells at the tip form an embryonal mass (Fig. 1.79C, D).
In G. ula, the early development of the embryo up to the primary suspensor cells is almost similar to that of G. gnemon. The nucleus of the primary suspensor cell (Fig. 1.80A) divides to form two unequal nuclei, of which the smaller nucleus is cut-off by a thin wall. This cell is called peculiar cell which forms the embryo (Fig. 1.80B).
The peculiar cell divides twice forming a four-celled stage (Fig. 1.80C, D) which further divides transversely resulting into a 8-celled embryo (Fig. 1.80E). The embryonal mass increases in size by the further irregular divisions (Fig. 1.80F). Some cells of the embryonal mass adjacent to the primary suspensor elongate to form the secondary suspensor.
Irrespective of the pattern of formation of embryonal mass and secondary suspensor, the cell of the embryonal mass in Gnetum are small and compact with dense cytoplasm forming the embryo-proper. The cells of the secondary suspensor are thin-walled, uninucleate and highly vacuolated. Both the primary as well as secondary suspensors push the embryo deep inside the endosperm for the nourishment of the embryo.
At the tip of the embryonal cells, a stem tip with two lateral cotyledons is differentiated (Fig. 1.81 B). A root tip with a root cap also develops at the opposite end of the stem tip. Simultaneously, a hump-like structure called feeder is developed in-between the stem and root tips (Fig. 1.81B). Thus, a mature embryo is composed of a stem tip, two cotyledons, a large feeder and a root tip covered with root cap.
In Gnetum, polyembryony takes place in various ways. Each of the primary suspensor tube may develop an embryo, thus a large number of embryos are formed from a single zygote (Fig. 1.79C).
Sometimes additional embryos may develop due to the proliferation of the proembryonal mass present at the tip of the secondary suspensor. Sometimes, the primary suspensor tube branches giving rise to several primary suspensor tubes, each of which may develop an embryo at its tip.
Seeds:
Gnetum seeds are oval in shape (Fig. 1.81 A) and green to brown-red in colour. The seeds remain covered with a three-layered envelop, of which outer is fleshy, middle is stony and inner is pepary. The nucellus is used up and the embryo is embedded within the endosperm. Gnetum shows one-year reproductive cycle where pollination, fertilisation and development of embryo take place in one year.
The germination of seed is epigeal. The seeds of G. ula germinate after one year’s of res-ting phase.
Figure 1.82 shows the life cycle of Gnetum.