Read this article to learn about the features, principle and types of biosensors.
A biosensor is an analytical device containing an immobilized biological material (enzyme, antibody, nucleic acid, hormone, organelle or whole cell) which can specifically interact with an analyte and produce physical, chemical or electrical signals that can be measured. An analyte is a compound (e.g. glucose, urea, drug, pesticide) whose concentration has to be measured.
Biosensors basically involve the quantitative analysis of various substances by converting their biological actions into measurable signals. A great majority of biosensors have immobilized enzymes. The performance of the biosensors is mostly dependent on the specificity and sensitivity of the biological reaction, besides the stability of the enzyme.
General Features of Biosensors:
A biosensor has two distinct components (Fig. 21.13).
1. Biological component—enzyme, cell etc.
2. Physical component—transducer, amplifier etc.
The biological component recognises and interacts with the analyte to produce a physical change (a signal) that can be detected, by the transducer. In practice, the biological material is appropriately immobilized on to the transducer and the so prepared biosensors can be repeatedly used several times (may be around 10,000 times) for a long period (many months).
Principle of a Biosensor:
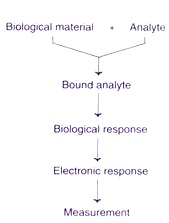
The desired biological material (usually a specific enzyme) is immobilized by conventional methods (physical or membrane entrapment, non- covalent or covalent binding). This immobilized biological material is in intimate contact with the transducer. The analyte binds to the biological material to form a bound analyte which in turn produces the electronic response that can be measured.
In some instances, the analyte is converted to a product which may be associated with the release of heat, gas (oxygen), electrons or hydrogen ions. The transducer can convert the product linked changes into electrical signals which can be amplified and measured.
Types of Biosensors:
There are several types of biosensors based on the sensor devices and the type of biological materials used. A selected few of them are discussed below.
Electrochemical Biosensors:
Electrochemical biosensors are simple devices based on the measurements of electric current, ionic or conductance changes carried out by bio electrodes.
Amperometric Biosensors:
These biosensors are based on the movement of electrons (i.e. determination of electric current) as a result of enzyme-catalysed redox reactions. Normally, a constant voltage passes between the electrodes which can be determined. In an enzymatic reaction that occurs, the substrate or product can transfer an electron with the electrode surface to be oxidised or reduced (Fig. 21.14).
This results in an altered current flow that can be measured. The magnitude of the current is proportional to the substrate concentration. Clark oxygen electrode which determines reduction of O2, is the simplest form of amperometric biosensor. Determination of glucose by glucose oxidase is a good example.
In the first generation amperometric biosensors (described above), there is a direct transfer of the electrons released to the electrode which may pose some practical difficulties. A second generation amperometric biosensors have been developed wherein a mediator (e.g. ferrocenes) takes up the electrons and then transfers them to electrode. These biosensors however, are yet to become popular.
Blood-glucose biosensor:
It is a good example of amperometric biosensors, widely used throughout the world by diabetic patients. Blood- glucose biosensor looks like a watch pen and has a single use disposable electrode (consisting of a Ag/AgCI reference electrode and a carbon working electrode) with glucose oxidase and a derivative of ferrocene (as a mediator). The electrodes are covered with hydrophilic mesh guaze for even spreading of a blood drop. The disposable test strips, sealed in aluminium foil have a shelf-life of around six months.
An amperometric biosensor for assessing the freshness of fish has been developed. The accumulation of ionosine and hypoxanthine in relation to the other nucleotides indicates freshness of fish-how long dead and stored. A biosensor utilizing immobilized nucleoside phosphorylase and xanthine oxidase over an electrode has been developed for this purpose.
Potentiometric Biosensors:
In these biosensors, changes in ionic concentrations are determined by use of ion- selective electrodes (Fig. 21.15). pH electrode is the most commonly used ion-selective electrode, since many enzymatic reactions involve the release or absorption of hydrogen ions. The other important electrodes are ammonia-selective and CO2 selective electrodes.
The potential difference obtained between the potentiometric electrode and the reference electrode can be measured. It is proportional to the concentration of the substrate. The major limitation of potentiometric biosensors is the sensitivity of enzymes to ionic concentrations such as H+ and NH+4.
Ion-selective field effect transistors (ISFET) are the low cost devices that can be used for miniaturization of potentiometric biosensors. A good example is an ISFET biosensor used to monitor intra-myocardial pH during open-heart surgery.
Conduct Metric Biosensors:
There are several reactions in the biological systems that bring about changes in the ionic species. These ionic species alter the electrical conductivity which can be measured. A good example of conduct metric biosensor is the urea biosensor utilizing immobilized urease. Urease catalyses the following reaction.
The above reaction is associated with drastic alteration in ionic concentration which can be used for monitoring urea concentration. In fact, urea biosensors are very successfully used during dialysis and renal surgery.
Thermometric Biosensors:
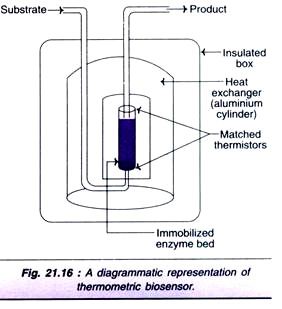
Several biological reactions are associated with the production of heat and this forms the basis of thermometric biosensors. They are more commonly referred to as thermal biosensors or calorimetric biosensors. A diagrammatic representation of a thermal biosensor is depicted in Fig. 21.16. It consists of a heat insulated box fitted with heat exchanger (aluminium cylinder).
The reaction takes place in a small enzyme packed bed reactor. As the substrate enters the bed, it gets converted to a product and heat is generated. The difference in the temperature between the substrate and product is measured by thermistors. Even a small change in the temperature can be detected by thermal biosensors.
Thermometric biosensors are in use for the estimation of serum cholesterol. When cholesterol gets oxidized by the enzyme cholesterol oxidase, heat is generated which can be measured. Likewise, estimations of glucose (enzyme-glucose oxidase), urea (enzyme-urease), uric acid (enzyme-uricase) and penicillin G (enzyme-P lactamase) can be done by these biosensors. In general, their utility is however, limited. Thermometric biosensors can be used as a part of enzyme-linked immunoassay (ELISA) and the new technique is referred to as thermometric ELISA (TELISA).
Optical Biosensors:
Optical biosensors are the devices that utilize the principle of optical measurements (absorbance, fluorescence, chemiluminescence etc.). They employ the use of fibre optics and optoelectronic transducers. The word optrode, representing a condensation of the words optical and electrode is commonly used. Optical biosensors primarily involve enzymes and antibodies as the transducing elements.
Optical biosensors allow a safe non-electrical remote sensing of materials. Another advantage is that these biosensors usually do not require reference sensors, as the comparative signal can be generated using the same source of light as the sampling sensor. Some of the important optical biosensors are briefly described hereunder.
Fibre optic lactate biosensor:
Fig. 21.17 represents the fibre optic lactate biosensor. Its working is based on the measurement of changes in molecular O2 concentration by determining the quenching effect of O2 on a fluorescent dye. The following reaction is catalysed by the enzyme lactate mono-oxygenase.
The amount of fluorescence generated by the dyed film is dependent on the O2. This is because O2 has a quenching (reducing) effect on the fluorescence. As the concentration of lactate in the reaction mixture increases, O2 is utilized, and consequently there is a proportionate decrease in the quenching effect. The result is that there is an increase in the fluorescent output which can be measured.
Optical Biosensors for Blood Glucose:
Estimation of blood glucose is very important for monitoring of diabetes. A simple technique involving paper strips impregnated with reagents is used for this purpose. The strips contain glucose oxidase, horse radish peroxidase and a chromogen (e.g. toluidine). The following reactions occur.
The intensity of the colour of the dye can be measured by using a portable reflectance meter. Glucose strip production is a very big industry worldwide.
Colorimetric test strips of cellulose coated with appropriate enzymes and reagents are in use for the estimation of several blood and urine parameters.
Luminescent biosensors to detect urinary infections:
The microorganisms in the urine, causing urinary tract infections, can be detected by employing luminescent biosensors. For this purpose, the immobilized (or even free) enzyme namely luciferase is used. The microorganisms, on lysis release ATP which can be detected by the following reaction. The quantity of light output can be measured by electronic devices.
Other Optical Biosensors:
Optical fibre sensing devices are in use for measuring pH, pCO2 and pO2 in critical care, and surgical monitoring.
Piezoelectric Biosensors:
Piezoelectric biosensors are based on the principle of acoustics (sound vibrations), hence they are also called as acoustic biosensors. Piezoelectric crystals form the basis of these biosensors. The crystals with positive and negative charges vibrate with characteristic frequencies. Adsorption of certain molecules on the crystal surface alters the resonance frequencies which can be measured by electronic devices. Enzymes with gaseous substrates or inhibitors can also be attached to these crystals.
A piezoelectric biosensor for organophosphorus insecticide has been developed incorporating acetylcholine esterase. Likewise, a biosensor for formaldehyde has been developed by incorporating formaldehyde dehydrogenase. A biosensor for cocaine in gas phase has been created by attaching cocaine antibodies to the surface of piezoelectric crystal.
Limitations of Piezoelectric Biosensors:
It is very difficult to use these biosensors to determine substances in solution. This is because the crystals may cease to oscillate completely in viscous liquids.
Whole Cell Biosensors:
Whole cell biosensors are particularly useful for multi-step or cofactor requiring reactions. These biosensors may employ live or dead microbial cells. A selected list of some organisms along with the analytes and the types of biosensors used is given in Table 21.8
Advantages of microbial cell biosensors:
The microbial cells are cheaper with longer half-lives. Further, they are less sensitive to variations in pH and temperature compared to isolated enzymes.
Limitations of microbial cell biosensors:
The whole cells, in general, require longer periods for catalysis. In addition, the specificity and sensitivity of whole cell biosensors may be lower compared to that of enzymes.
Immuno-Biosensors:
Immuno-biosensors or immunochemical biosensors work on the principle of immunological specificity, coupled with measurement (mostly) based on amperometric or potentiometric biosensors. There are several possible configurations for immuno-biosensors and some of them are depicted in Fig. 21.18, and briefly described hereunder.
1. An immobilized antibody to which antigen can directly bind (Fig. 21.18A).
2. An immobilized antigen that binds to antibody which in turn can bind to a free second antigen (Fig. 21.18B).
3. An antibody bound to immobilized antigen which can be partially released by competing with free antigen (Fig. 21.18C).
4. An immobilized antibody binding free antigen and enzyme labeled antigen in competition (Fig. 21.18D).
For the biosensors 1-3, piezoelectric devices can be used. The immuno-biosensors using enzymes (4 above, Fig. 21.18D) are the most commonly used. These biosensors employ thermometric or amperometric devices. The activity of the enzymes bound to immuno-biosensors is dependent on the relative concentrations of the labeled and unlabeled antigens. The concentration of the unlabeled antigen can be determined by assaying the enzyme activity.