Chloroplasts are organelles found in the green tissues of plants and are responsible for the absorption of light energy, the synthesis of carbohydrates, and the evolution of molecular oxygen.
The sum of these three processes is called photosynthesis. Light energy captured by the chloroplast is converted into potential chemical energy in the form of carbohydrates and starts the “energy chain” in nature.
The oxygen evolved during the capture of light energy becomes the ultimate oxidizing agent for cellular metabolic reactions.
Mitochondrial oxidations, as well as other oxidations in plant, animal, and many microbial cells, depend on this primary source of oxygen. It is currently believed that the entire supply of oxygen in the atmosphere today was derived from and is presently maintained by photosynthesis.
A chloroplast is any membrane-encased organelle containing chlorophyll that belongs to a group of related organelles in plants called plastids. The plastids have a variety of morphological forms, carry out diverse functions, and store many different compounds. For example, the amyloplast is the starch-storing plastid of potato tubers, and the chromoplast is the lycopene-containing plastid that gives the fruit of tomatoes its red color. Each of the diverse plastids is believed to arise from a common proplastid precursor. All the major groups of plants, with the exception of the fungi, contain chloroplasts.
There are two fundamental types of chloroplasts each associated with one of two types of photosynthesis: C3 photosynthesis and C4 photosynthesis. C3 photosynthesis converts (or “fixes”) carbon dioxide into three-carbon acids, whereas C4 photosynthesis fixes CO2 into four-carbon acids. Plants that carry out C4 photosynthesis (called C4 plants) are generally grasses or plants that are endemic to environments with little water.
C3 plants (those that carry out C3 photosynthesis) are commonly broad-leafed flowering plants, cone-bearing plants, or those living in areas of adequate moisture. The characteristics of these two types of photosynthesis and their associated chloroplast types. There may be a single chloroplast or dozens of chloroplasts in a cell. Frequently, the simpler plants, such as the algae, contain only one or a few chloroplasts per cell, whereas vascular plants, such as the cone-bearing and flowering plants, have many chloroplasts in each cell.
Photosynthesis occurs in some prokaryotic organisms such as the cyanobacteria (or blue-green algae) and the anaerobic photosynthetic bacteria. These prokaryotes do not have true chloroplasts; instead, they have lamellated structures called chromatophores that carry out only the light-absorbing reactions of photosynthesis but not the carbohydrate-synthesizing reactions.
For many laboratory studies of photosynthesis, the single-celled plant Chlorella is employed. This alga has one cup-shaped chloroplast that practically fills the cell. The organism can be easily and conveniently cultured with artificial lighting in the laboratory in solutions of inorganic salts. Because of the one-to-one relationship between cell and chloroplast and the ease with which the cells can be grown and enumerated, quantitative studies using chloroplast preparations of known content are possible.
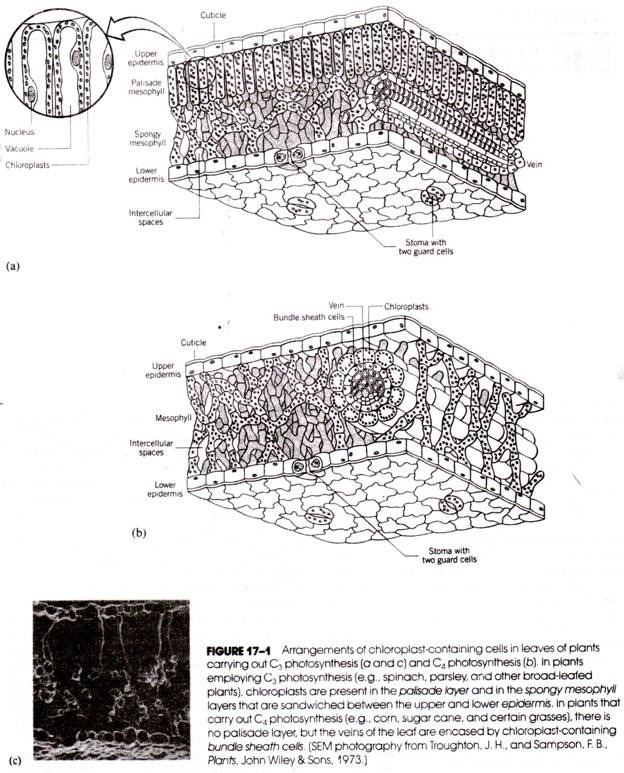
For studies with chloroplasts of higher plants, leaves are generally used, and spinach and parsley leaves are probably the most popular source. In these leaves, the chloroplasts are found in greatest numbers in two internal tissues, the palisade mesophyll and the spongy mesophyll (Fig. 17-1a). Both tissues lie between an upper and lower epidermis and have thin cell walls that are easily broken. The number of chloroplasts in each cell varies within an organism with changing environmental conditions and varies greatly from one species to another. In spinach, there are between 20 and 40 chloroplasts in each palisade parenchyma cell.
In the palisade parenchyma, the chloroplasts lie along the edges of the cell, the center of the cell being filled with large vacuoles. In the spongy parenchyma, the chloroplasts are more randomly distributed throughout the cytoplasm of the cell.
In many genera, cytoplasmic streaming (i.e., cyclosis) moves the chloroplasts about the cell, and in a few instances an active amoeboid-type of movement by chloroplasts has been observed. Their positions in the cell and movement about the cell maximize the exposure of the chloroplasts to light. Chloroplasts are routinely isolated from plant tissues by differential centrifugation following the disruption of the cells. Leaves are homogenized in an ice- cold buffered isotonic saline solution (e.g., 0.35 M NaCl) at pH 8.0. The disruption is generally carried out with bursts of a Waring blender.
After filtration through a nylon gauze (20 μm pore size) to remove the larger particles of debris (cell nuclei, tissue fragments, and unbroken cells), the chloroplasts are separated by centrifugation at 200 g for 1 minute. The chloroplast-rich pellet is then re-suspended and centrifuged again at 2000 g for 45 seconds to re-sediment the chloroplasts.
Chloroplast preparations obtained by this procedure are generally mixtures of intact and broken organelles. Because the chemical composition, rate of photosynthetic activity, and other properties of intact chloroplasts differ significantly from those of damaged organelles, it is often desirable to separate the two populations. This may be accomplished by rate or isopycnic density gradient centrifugation of the chloroplast preparation.
Chloroplast size is quite variable. Although the average diameter of a chloroplast in higher plant cells is between 4 and 6 μm, the size may fluctuate according to the amount of available illumination. In sunlight, chlorophyll is more readily synthesized by the plant, and the chloroplasts increase in size; in the shade, chlorophyll synthesis declines, and there is a corresponding reduction in chloroplast size.
Changes in chloroplast shape are also observed after short-term exposure of plants to light. Short-term light exposure produces a small but measurable decrease in chloroplast volume. Presumably, this is due to a light- induced production of ATP, for the addition of ATP to chloroplasts in the dark causes a reduction in volume. Polyploid cells contain larger chloroplasts than comparable diploid cells.
The shape of most chloroplasts in higher plants is spheriod, ovoid, or discoid (Fig. 17-2). Other irregular shapes sometimes occur but are more common in lower plants. For example, in algae, cup-shaped, spiral, star-shaped, and digitate forms of chloroplasts are observed. The shape and structure of chloroplasts can also be altered by the presence of starch granules. During periods of active photosynthesis, the sugars formed in the chloroplasts are polymerized into starches that precipitate as small granules. The starch granules are usually ellipsoidal and may be up to1.5µm long.
Fine Structure of the Chloroplast:
The chloroplasts of C3 plants are composed of two membrane layers similar to those of mitochondria. Each membrane is about 50 A thick and the two membranes are separated by a space of about 70-100 A. The outer membrane, which lacks folds or projections, serves to delimit the organelle and regulate the transport of materials between the cytoplasm and the interior of the organelle. The inner membrane parallels the outer membrane, but inward folds of this membrane are extensive. The inner membrane gives rise to a series of internal parallel membrane sheets called lamellae (Fig. 17-3). The lamellae are suspended in a granular fluid or matrix that appears somewhat electron-dense in electron photomicrographs. This matrix is referred to as the stroma. The lamellae form a complex series of membranes through the stroma.
Most of the lamellae in the chloroplasts of higher plants are organized to form disk-shaped sacs called small thylakoids. The small thylakoids are often arranged in stacks called grana (one stack is a granum) having diameters of 300 to 600 nm. Because the thylakoids are disk-shaped, the grana appear much like a stack of coins (Figs. 17-3a and 17-3b).
A typical chloroplast has between 40 and 60 grana, and each granum may be composed of 2 to 100 small, flattened thylakoids. Frequently, a small portion of the thylakoid extends radially into the stroma forming a branching tube, or large thylakoid that communicates with other small thylakoids and grana. Collectively, the branching and anastomosing network is called the stroma lamellae.
Structure of the Thylakoid:
The adjacent membranes of neighboring thylakoids within each of the grana form thick layers called grana lamellae (Fig. 17-3). Electron photomicrographs of grana lamellae fixed with glutaraldehyde and stained with osmium reveal a five-layered arrangement consisting of three dark 40-Å-thick osmiophilic layers enclosing two 17-Å-thick osmiophobic spaces. Freeze-fracture techniques indicate that the grana membranes contain numerous particles.
The particles, which are primarily protein in composition, appear to be of two basic sizes, 105 and 140 A in diameters (Fig. 17-4). The stroma lamellae contain mainly the smaller-size particles. The larger particles are abundant on the EF face of the grana membrane and are associated with photosystem II of photosynthesis (described later in the chapter). The smaller particles are associated with photosystem I and are present in about equal numbers on the PF face of both the grana and stroma membranes (Fig. 17-5).
Stroma Structures:
The granular stroma contains a variety of particles. The presence of starch granules was noted earlier. Electron micrographs also reveal a number of osmiophilic granules and groups of ellipsoidal structures called stromacenters. Strands of DNA and ribosomes are also scattered through the stroma. The chloroplasts of C4 plants have the same general structures as those of C3 plants (an outer enclosing membrane, grana and stroma thylakoids, a stroma with DNA, ribosomes, and stromacenters), but there are some notable differences.
The C4 plant chloroplasts all have a peripheral reticulum, which is a group of anastomosing tubules about the periphery. In many C4 plants such as corn there are two types of chloroplasts. Those found in the mesophyll cells (see Fig. 17-1b) are similar to the chloroplasts of C3 plants but lack starch grains. The chloroplasts found in the cells surrounding the leaf veins (called bundle sheath cells) contain starch granules but have elongated grana.
Chemical Composition of Chloroplasts:
The organic constituent present in greatest quantity in the chloroplast is protein, which may represent up to 70% of the dry weight (Table 17-1). In leaf cells, 75% of the total cell nitrogen is found within the chloroplasts. Both structural and soluble proteins have been identified, but only a few of these have been extracted and purified.
A peptide analysis by SDS gel electrophoresis shows compositional differences between stroma and grana lamellae, but the differences are primarily quantitative rather than qualitative. The relative compositions of stroma lamellae and grana lamellae are shown in Table 17-2.
Essentially all the pigments and cytochromes are located in the lamellae. The stroma lacks these com-pounds but contains DNA and RNA, which are not present in the lamellae. Most of the RNA is associated with the ribosomes of the stroma. The amount of DNA is low; estimates are 10-15 to 10-14 grams per chloroplast or about 0.03% of its dry weight. However, this is enough information to account for the synthesis of some chloroplast proteins, including some of the enzymes of photosynthesis. The disposition of chloroplast DNA during chloroplast division is unclear.
Lipid and lipid-soluble pigments account for about 34% of the dry weight of the spinach chloroplast. An exceedingly large number of different lipid compounds have been identified. The more common lipids are the galactosyl diglycerides, phospholipids, quinones (including vitamin K), and sterols.
The Chlorophylls:
The green pigments of chloroplasts and the main sources of the color of green plants are the chlorophylls. Although a large number of chemically distinct chlorophylls have been identified in a variety of different plants, the structures of these chlorophylls are basically the same. (The structures of chlorophylls a and b are given in Fig. 17-6.) It is customary to identify each chlorophyll by a different letter. All photo- synthetic plants have been found to contain chlorophyll a, but the presence of the secondary chlorophylls b, c, d, and e depends on the type of plant.
Higher plants usually have chlorophyll b. In the photosynthetic bacteria, a chlorophyll called bacteriochlorophyll occurs in place of chlorophyll a. Together, chlorophylls a and b represent about 8% of the dry weight of spinach chloroplasts, with an a:b weight ratio of 2.1 to 3.5. In most plants, the a:b ratio varies according to the light intensity to which the plants are exposed. For example, alpine plants, which receive light of high intensity, have an average ratio of 5.5. The ratio is 2.3 in shade plants.
Each chlorophyll has a characteristic light absorption spectrum. The in vitro light absorption spectra of chlorophylls a and 6 are shown in Figure 17-7. Extracted chlorophyll a has absorption maxima at 430 and 670 nm, whereas the absorption maxima of chlorophyll b occur at 455 and 640 nm.
In vitro absorption maxima of other plant and bacterial pigments are indicated in Table 17-3. The absorption spectrum, and maxima of plant pigments vary according to the solvent used for extraction. Therefore, it is not surprising that values obtained during in vivo measurements differ from those yielded by extracts. For example, in vivo studies of chlorophyll a indicate that its native absorption maximum occurs at 677 nm. One very important form of chlorophyll a that is readily bleached by light has an absorption maximum at 700 nm. This form, which represents only about 0.1% of the total chlorophyll a molecules present in a sample, is called P700 or chlorophyll a1.
The Carotenoids:
The carotenoids are all long-chain isoprenoid compounds having an alternating series of double bonds. Although these compounds are synthesized only in plant tissue and participate in photosynthesis, they also serve as precursors of vitamin A in animal tissues. Most carotenoids are yellow, orange, or red. The formulas of a, (3, and 7-carotene are shown in Figure 17-8, and their absorption maxima are given in Table 17-3. Most of these pigments are located in the chloroplast lamellae and are believed to function as accessory pigments for light absorption during photosynthesis.
Location and Arrangement of the Pigments:
Both the chlorophylls and the carotenoids are located almost exclusively in the chloroplast lamellae. The lamellae are about half protein and half lipid, the two pigments residing primarily in the lipid component. Some lipid is also represented by the osmiophilic granules of the stroma, but these are not believed to contain chlorophyll. Because each chlorophyll molecule has a hydrophilic portion (the tetrapyrrole) and a lipophilic portion (the phytyl chain), the chlorophyll molecules are thought to be aligned in a specific manner within the lamellae. The pyrrole groups form weak bonds with the lamellar protein. The carotenoids are dissolved in the lipid adjacent to the chlorophyll molecules.
The chloroplast stroma contains many of the enzymes associated with photosynthesis. Chloroplast protein synthesis also takes place in the stroma. Circular DNA strands about 40 μm long have been isolated from the chloroplast along with ribosomes and polyribosomes. The DNA strands have 115-200 kbp (kilo- base pairs) and contain genes for about 180-280 polypeptides. Chloroplast ribosomes belong to the 70 S class and contain 23 S and 16 S RNA; thus, they are smaller than those found in the cytoplasm of plant (and animal) cells.
Development of Chloroplasts:
New chloroplasts are produced by the division of mature chloroplasts (Fig. 17-9) or by development from pro-plastids in the cell. The cells of young shoots of higher plants may contain 20 to 40 of these small ovoid bodies (Fig. 17-10). Apparently, pro-plastids can develop into a number of different plastid types in addition to chloroplasts. As a pro-plastid develops into a chloroplast, its inner membrane gives rise to infoldings that form the lamellae and thylakoids. Pro-plastids, as well as mature chloroplasts, increase in numbers by a form of division (Fig. 17-10b).
The control of chloroplast development is not fully understood. The differentiation of new organelles appears to rely on an interaction between genetic information present in the cell nucleus and information present in the chloroplast itself. Chloroplasts contain DNA that is transcribed and translated within the organelle to form some of the chloroplast proteins; however, other chloroplast proteins are derived from transcription and translation of nuclear DNA.
The intriguing but controversial hypothesis that chloroplasts are the evolutionary products of bacteria like organisms that “invaded” eukaryotic cells and established a symbiotic relationship with the host cell is similar to the proposal for the origin of mitochondria.
In the case of the chloroplast, the host could have been a heterotrophic cell. Interestingly, chloroplasts are found in the cells of a subgroup of nudibranchs (marine snails lacking a shell) that feed on algae. As the algae cell cytoplasm passes through the intestine of the nudibranch, whole chloroplasts are absorbed into the tissue and may persist there for the life of the animal. When the animal is in light, the absorbed chloroplasts evolve oxygen.