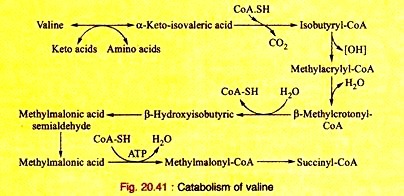
In this article, we consider some of the fundamental principles that govern the passive movements of water, ions, and various other molecules through cell membranes.
The term passive is intended to denote that the movement of the substance through the membrane is not associated with any chemical or metabolic activities in the membrane.
Passage through the membrane in such instances is regulated by such factors as the concentration gradient across the membrane and the chemical and physical relationships between the membrane and substances inside and outside the cell.
Later, we will direct our attention to movements through the membrane that are accompanied by chemical changes, metabolic activity, or gross molecular rearrangements within the membrane itself.
1. Osmosis and Diffusion across Membranes:
Membranes that allow substances to pass through them are said to be permeable to the substance. Nearly all plasma membranes are permeable to water. If water (or some other solvent) is-the only substance that can pass through the membrane, the membrane is said to be semipermeable. Membranes that display a gradation of permeability to water and dissolved solutes (i.e., membranes that permit water to pass through more readily than salts, sugars, etc.) are said to be selectively permeable.
Water molecules are continuously moving into and out of cells through the plasma membrane. Such movements are generally not discernible as changes in cell size or shape because the flux in each direction is the same. When the concentrations of solutes inside and outside the cell differ, the water flux in one direction may be greater than in the other direction, and the cell may swell or shrink.
Water moves from a region of low solute concentration to a region of higher solute concentration in order to establish concentration equilibrium. The movement of water (or some other solvent) in response to such a solute concentration gradient is known as osmosis.
Osmosis may readily be demonstrated using artificial membranes such as cellophane, which is permeable to small molecules such as salts, sugars, and amino acids, but is impermeable to large molecules such as proteins. If a cellophane bag filled with a concentrated salt solution is connected to a vertical length of glass tubing and is then immersed in a container of distilled water, water will pass into the bag by osmosis.
The entry of water into the cellophane bag will cause the level of water in the glass tubing to rise (Fig. 15-31). Salts permeate cellophane membranes more slowly than water, so that some time elapses before the salt molecules pass out of the bag into the surrounding water.
The movement of solute molecules (in this case, salt molecules) from a region of high concentration (inside the bag) to one of lower concentration (outside the bag) occurs by the process of diffusion. In the case illustrated in Figure 15-31, the initial movement of water into the cellophane bag is followed by the outward diffusion of salt from the bag.
As the solute concentration inside the bag decreases, the liquid level in the glass tubing falls as water molecules leave the bag by osmosis. The movements of salt and water molecules continue until the salt concentrations inside and outside the bag are equal.
Consider a case in which the cellophane bag is filled with a solution containing an impermeable solute. As in the previous case, water will enter the bag by osmosis, causing the liquid level in the glass tubing to rise. Because the solute is impermeable, it cannot diffuse from the bag, and concentration equilibrium across the membrane cannot be achieved.
Consequently, water will continue to enter the bag and rise in the glass tubing until a height is reached at which the pressure at the base of the water column is just great enough to prevent any further water influx. In theory, the water will remain at this level indefinitely.
The pressure that is created inside the bag by the impermeable solute and that supports the column of water is called osmotic pressure. Its numerical value can be approximated by measuring the height of the water column. Usually osmotic pressure is expressed in millimeters of mercury (i.e., mm Hg) rather than in inches of water. Devices that are used to measure osmotic pressure are called osmometers (Fig. 15-32).
2. Osmosis and Diffusion across Cell Membranes:
Cellular phenomena associated with osmosis and diffusion across the plasma membrane are readily demonstrated using red blood cells, sea urchin eggs, or certain plant cells. The plasma in which the red blood cells are normally suspended contains the same concentration of impermeable salt (0.15 M NaCl) as the erythrocyte cytoplasm (0.15 M KCl).
Normal plasma is said to be isotonic to the red cell. If the plasma is diluted with water, its salt concentration will decrease, and the plasma will become hypotonic to the red blood cell. Any suspending medium containing an impermeable solute concentration that is lower than the corresponding solute concentration in the cells suspended in that medium is considered hypotonic.
In the case of hypotonic plasma, water will enter the red cells by osmosis, causing the cells to swell. The same effect can be produced by placing red blood cells in any hypotonic solution. Just how much water will enter the cell depends on how hypotonic the suspending medium is.
For example, if the red cells are suspended in plasma containing one-half the normal salt concentration, water will enter the cells until they swell to twice their original volume. This will reduce the internal salt concentration to one-half its former value, bringing the internal and external salt concentrations into equilibrium (Fig. 15-33).
If the cells are suspended in a solution of even greater hypotonicity, then proportionately more water will have to enter the cell to reduce the internal salt concentration to that outside the cell. Obviously, cells can tolerate only a certain amount of swelling before the membrane ruptures, spilling the cell contents into the surrounding medium; this is called osmotic lysis.
Red blood cells lyse when suspended in very dilute salt solutions or in distilled water (Fig. 15-34). In the specific case of the red blood cell, this phenomenon is called hemolysis, because hemoglobin from the red blood cell is released into the suspending medium. Other animal cells behave in a similar manner in appropriately hypotonic media.
Plant cells generally do not lyse even when placed in distilled water because cell swelling is limited by the rather inflexible cellulose cell wall. In hypotonic solutions, plant cells swell as water enters the cytoplasmic vacuoles by osmosis. This forces the cytoplasm to the margins of the cell wall. Under these conditions, the plant tissue becomes turgid (Fig. 15-34). Equal concentrations of impermeable salts and non- dissociating (nonionizing) molecules (such as sucrose and other sugars) do not exhibit the same osmotic effects. For example, 0.15 M NaCl exerts twice the osmotic pressure as does 0.15 M sucrose.
This is because 0.15 M NaCl undergoes dissociation in water to produce twice the number of particles (i.e., ions) per cubic centimeter as 0.15 M sucrose. Thus, 0.15 M NaCl, 0.15 M KCl, 0.10 M CaCl2, and 0.3 M sucrose would all exert the same osmotic pressure, because they produce the same particle concentrations in water. Most cell membranes, including the plasma membrane of the red blood cell, are impermeable to sucrose. Therefore, both 0.15 M NaCl and 0.3 M sucrose are isotonic to red blood cells. Solutions that contain higher concentrations of impermeable solute than are found inside cells are said to be hypertonic.
What happens to red blood cells when they are placed in a hypertonic sucrose solution is depicted in Figure 15- 35. Water moves by osmosis from the cells into the medium until the concentration of solute inside and outside the cell is the same. The shrinkage associated with such water loss is called crenation. Other animal cells behave in a similar manner.
When plant cells are placed in hypertonic media they undergo plasmolysis, that is, water passes from the cytoplasmic vacuoles into the space between the cell wall and plasma membrane (Fig. 15-36).
So far, we have considered only the movement of water into and out of the cell. When the concentration of a permeable solute is greater outside a cell than inside, the solute molecules diffuse into the cell until the external and internal concentrations are in equilibrium. In most instances, the combined volume of all the cells present in a cell suspension is only a small fraction of the total volume of the suspending medium. Therefore, the quantity of solute entering (or leaving) the cells has a negligible effect on the solute concentration of the medium.
Consider what happens when red blood cells are placed in an isotonic saline solution (e.g., 0.15 M NaCl) containing 0.1 M urea. The red blood cell membrane is permeable to urea, and because red blood cells normally contain little or no urea, urea molecules will diffuse into the cell until the intracellular urea concentration reaches 0.1 M. Even after concentration equilibrium is achieved, urea molecules will continue to pass through the cell membrane into and out of the cell.
However, the migration in each direction will be the same, so that no net change in the urea concentration of the cell occurs. If the cells are transferred to an isotonic saline solution lacking urea, urea molecules will diffuse back out of the cell. Many substances enter and leave cells by diffusion through the plasma membrane.
The Permeability Constant:
The quantity of solute diffusing from one region to another depends on the concentration difference between the two regions, that is, the concentration gradient. Concentration gradients often exist between the internal and external environments of a cell. If the solute can permeate the cell membrane, diffusion in the direction of the concentration gradient into (or out of) the cell ensues.
The diffusion rate for a solute is given by Ficke’s equation:
Where
dS/dt = the number of moles of solute, S, diffusing from region 1 to region 2 in the time interval dt.
D = the diffusion coefficient of the solute. Each solute has a specific diffusion coefficient; the units of the diffusion coefficient are moles per unit cross-sectional area per unit concentration gradient per unit time. This reduces to the dimensions square centimeters per second. The diffusion coefficient of a solute is determined in part by the solute molecule’s size and shape.
A = the cross-sectional area through which diffusion occurs.
C1 and C2 = the concentrations of S in regions 1 and 2, respectively.
x= the distance between regions 1 and 2.
C1 –C2/x = the concentration gradient.
In the case of diffusion into or out of the cell, we may let
x = the membrane thickness.
C1 = Cout = the concentration of S outside of the cell.
C2 = Cin = the concentration of S inside of the cell.
A new term, the permeability constant, K, which describes the diffusion of a particular substance across a particular membrane, may be substituted for D/x in equation 15-1 to yield
The dimensions of K are centimeters per second. Because the total amount of substance S in a cell of volume V is VCin, substitution of VdCin for dS in equation 15-2 followed by a transposition yields
Thus, it can be seen that other things being equal, the rate of change of the internal solute concentration (i.e., dCin/dt) depends on the surface area to volume ratio of the cell (i.e., A/V). Because the ratio A/V is at a minimum for spherical objects, the rate at which solute is exchanged by diffusion between a cell and its environment is increased by deviation from spherical shape.
If the external volume is sufficiently large in comparison with the cell volume (this generally is the case), then Cout will not change significantly with time as a result of diffusion. That is, during the time interval between t0 and t1, Cout will remain constant.
Therefore, equation 15- 3 may be solved by integration as follows:
Cout Qin, A, V, and ∆t may be determined experimentally, so that equation 15-6 can be employed to find the permeability constant. Evaluation of the permeability constants for a variety of substances .entering a cell permits much more to be learned about the chemical nature and behavior of the cell membrane. The permeability constants of glycol, urea, glycerol, and sucrose for the membranes of the red blood cell, the bacterium Beggiatoa, and the alga Chara are compared in Table 15-5. The marked differences that can be seen in these values reflect the variation that must exist in the composition and organization of the plasma membranes of these cells.
Factors Influencing Permeability:
A number of different molecular parameters influence the ability of a substance to permeate cell membranes. Important among these are the distribution coefficient, molecular size, and charge. The distribution coefficient of a substance relates its solubility in oil to its solubility in water. In general, the more non-polar a substance, the more soluble it is in oil and the less soluble it is in water. In contrast, the more polar a substance is, the less soluble it is in oil and the more soluble it is in water.
One of the most extensive studies of the relationship between distribution coefficient and membrane permeability was that carried out in the 1930s by R. Collander and H. Barlund using the unicellular alga Chara. They found that, in general, the higher the distribution coefficient of a compound, the greater its permeability to the cell membrane. Overton had made similar but less quantitative observations many years earlier, and this led him to propose that the cell membrane was composed of lipid.
The relationship between lipid solubility and membrane permeability is the basis for the proposal that lipid-soluble substances readily pass into or out of the cell by dissolving through lipid regions of the cell membrane. For chemically related substances, permeability increases with increasing distribution coefficient regardless of molecular size. However, in general, when two molecules have similar distribution coefficients, the smaller molecule is more permeable than the larger molecule.
The fact that water has a very high permeability constant in spite of its low lipid solubility led to the suggestion that there are hydrophilic pores in the cell membrane through which small polar molecules may more readily diffuse. The permeability constant for water is about 1 x 10-4 cm/sec.
This value is only 0.001% of the rate at which water molecules diffuse across a water layer the thickness of the cell membrane. This implies that if they are present, hydrophilic pores must cover only a small percentage of the surface area of the cell. In the case of the red blood cell, A. K. Solomon has estimated that 0.06% of the surface area is occupied by such pores.
In addition to the distribution coefficient, the effective size of a molecule influences its membrane permeability. In general, small molecules are more permeable than large molecules. Substances of very high molecular weight (such as starch, glycogen, and many proteins) are usually unable to permeate the membrane at all. This is not to imply that cells cannot incorporate or eliminate very large molecules. Indeed, they do; however, the process involves mechanisms other than diffusion.
Electrolytes enter cells more slowly than do nonelectrolytes of similar effective molecular size, and strong electrolytes enter more slowly than weak electrolytes. Because pH influences the degree of ionization of many electrolytes, permeability to electrolytes varies with the pH. In general, monovalent ions (e.g., Na+, K+, I–, CI–) permeate membranes more readily than divalent ions (e.g., Ca2+, Mg2+, SO42-), which in turn are more permeable than trivalent ions (e.g., Fe3+). The relative permeability of the membrane to ions of the same valency number but different sign (i.e., K+ versus CI–) depends on the particular cell considered.
Not all ions of the same valency and sign are equally permeable. Most differences can be explained in terms of the relative effective sizes of the ions in an aqueous medium. The charge on the ion attracts neighboring water molecules, causing them to align themselves around the ion to form spheres of hydration. The effective size of the ion is therefore determined by the number of these hydration spheres.
Ions of low molecular or atomic weight attract more water molecules than do ions of higher molecular weight because there are fewer electron shells around the atomic nuclei to neutralize the ionic charge. Accordingly, the effective size of Li+ (atomic number=3) is greater than that of Na+ (atomic number = 19) and so on. Therefore, membranes are usually more permeable to K+ than to Na+, and they are more permeable to Na+ than to Li+.
Ions may enter or leave a cell through pores in the plasma membrane or the endoplasmic reticulum. Cations are attracted to and pass more readily through pores lined by negative charges (e.g., negatively charged R-groups of amino acids of membrane proteins, etc.). Anions enter or leave the cell through positively charged pores.
3. The Gibbs-Donnan Effect:
Proteins behave like ions because the R-groups of their amino acids may bear positive or negative charges. The net ionic charge of the protein molecule depends on the relative numbers of positive and negative side groups. Most soluble proteins behave like an- ions because they possess more negative than positive sites.
Unlike many other ions, proteins are generally too large to permeate cell membranes. The Gibbs- Donnan effect (after J. W. Gibbs and F. G. Donnan) describes the effect that proteins have on the equilibrium distributions of small ions across a selectively permeable membrane.
When a selectively permeable membrane separates an electrolyte solution containing proteins from one that lacks proteins, the concentrations reached at equilibrium for each permeable ionic species will not be the same on both sides of the membrane. Instead, the concentration of small ions having the same sign as the protein will be lower on the side of the membrane containing the protein (i.e., inside the cell) and the concentration of small ions of opposite sign will be higher.
The Gibbs-Donnan effect does not influence the equilibrium distributions of nonionized substances such as glucose and urea. The Gibbs-Donnan phenomenon is responsible for the rupture and lysis of bacteria and other organisms when antibodies attach to their surface.
The reason is that the binding of antibody sets off the “complement” reactions in which a succession of plasma proteins (in a cascade-type activation) form enzymes that puncture a hole in the invading bacterial cell’s membrane. This – is followed by an inrush of ions and water that leads to cell lysis.
The Gibbs-Donnan Effect May Be Illustrated as Follows Suppose that two chambers of equal and fixed volumes are separated by a membrane permeable to water and small ions but impermeable to proteins. Into chamber 1 is placed a sodium proteinate (NaPr) solution of concentration C, and into chamber 2 a NaCl solution of concentration C2. Because the chloride ions are permeable and present at a greater concentration in chamber 2 than in chamber 1 (i.e., chamber 1 initially has no CI–), Cr will diffuse into chamber 1.
To preserve the electrical neutrality of each chamber, the diffusion of CI” into chamber 1 must be accompanied by the diffusion of an equivalent amount of Na+. Therefore, chamber 2 will be left with equal but reduced concentrations of Na+ and CI–.
According to the law of mass action, at equilibrium the products of the diffusable ion concentrations in each chamber will be equal. Therefore, if we let X equal the concentration of CI” diffusing from chamber 2 into chamber 1 (X will of course also equal the concentration of Na+ accompanying CI”), then at equilibrium
(C1 + X) (X) = (C2-X) (C2-X)
C1X + X2 = (C2)2 – 2C2X + X2 (15-7)
C1X = (C2)2-2C2X
And
Equation 15-8 is called the Gibbs-Donnan equilibrium equation and may be used to determine the equilibrium distribution of ions between the protein-containing and protein-free portions of the two-chamber system. Let us consider a specific case in which chamber 1 initially contains 0.01 M sodium proteinate (i.e., 0.01 M Na4 and 0.01 M Pr) and chamber 2 contains 0.03 M NaCl (i.e., 0.03 M Na+ and 0.03 M CI–).
The concentration of CI– (and Na+) diffusing across the membrane and into chamber 1 from chamber 2 may be determined using equation 15-8 as follows:
Therefore, the equilibrium distribution of ions would be (see also Fig. 15-37):
Note that:
(1) An electrical balance exists within each chamber,
(2) The osmotic pressure is greater in chamber 1 than in chamber 2 as a result of the higher total concentration of particles (no osmosis occurs because the chamber volumes are fixed), and
(3) There are more sodium ions but fewer chloride ions in chamber 1 than in chamber 2.
By extrapolating the observations made for the artificial two-chamber system just described to cells (i.e., chamber 1) and their surrounding milieu (i.e., chamber 2), it may be seen that the Gibbs-Donnan effect would result in an unequal distribution of permeable ions across the cell membrane, that is, a stable ionic concentration gradient would be established. Just such a phenomenon accounts in part for the resting electrochemical membrane potentials possessed by many cells.
In the cases we have considered so far, the chamber volumes have been equal and fixed. This, of course, is not necessarily the case for a cell and its surroundings. The flexibility of the plasma membrane permits notable fluctuations in cell shape and volume.
The greater osmotic pressure created inside the cell by the Gibbs-Donnan effect causes water to pass into the cell’ by osmosis, increasing the cell’s volume and lowering its ion concentration. Because this disturbs the Gibbs-Donnan equilibrium previously established, a new equilibrium is achieved by the passage of additional permeable ions into the cell. Osmosis halts when the hydrostatic pressure created by water inside the cell balances the osmotic pressure generated by the dissolved cell solute.