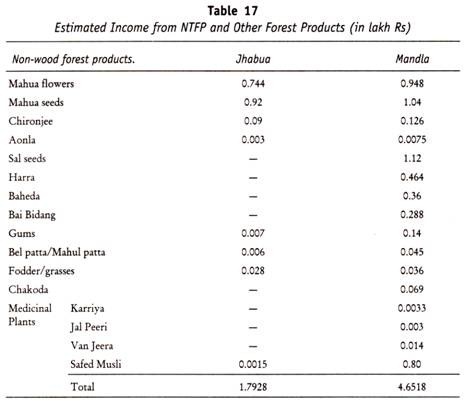
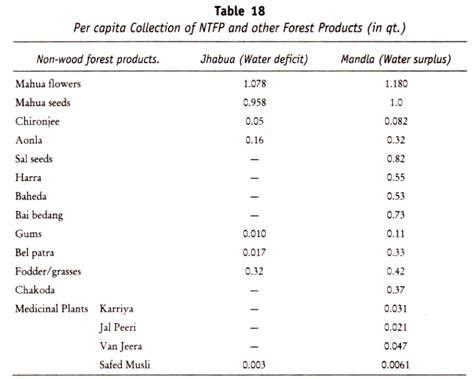
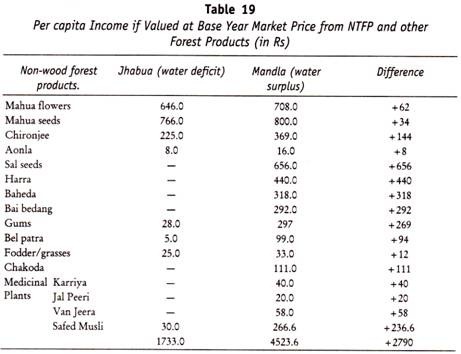
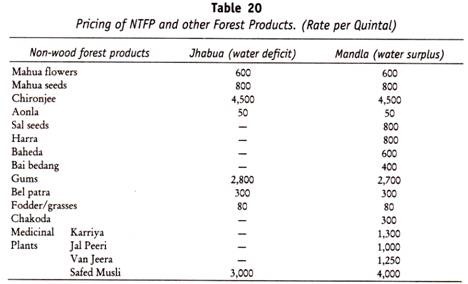
The following points highlight the three types of carbohydrates. The types are: 1. Monosaccharides 2. Disaccharides and 3. Polysaccharides.
Classification of Carbohydrates:
Contents
Carbohydrate Type # 1. Monosaccharides:
They are the sugar units that cannot be further hydrolysed into simpler units. There are two major classes of monosaccharide’s.
1. Aldoses:
Sugars containing an aldehydic group are known as aldoses, e.g., Glucose, galactose, mannose, ribose and glycerose.
2. Ketoses:
Sugars containing a ketonic group are known as ketoses. e.g., Dihydroxyacetone, fructose and seduloheptulose.
Depending upon the number of carbon atoms, aldoses and ketoses are further classified as:
Physical Characters of Monosaccharides:
1. Asymmetric carbon atom/chiral centre:
A carbon atom substituted by four different groups or atoms is known as asymmetric carbon atom. All carbohydrates except dihydroxyacetone have one or more asymmetric carbon atoms.
2. Isomers:
Two compounds having the same molecular formula but different structural formula are known as isomers. The number of isomers can be calculated from the number of chiral centres (n). The general formula is 2n. Glucose has four asymmetric carbon atoms, i.e., n = 4, so 24 = 16 isomers are possible for glucose.
(a) Epimers:
When sugars differ only in the configuration around one specific carbon atom they are called epimers. e.g., Glucose and mannose are epimers at C2 whereas glucose and galactose are epimers at C4.
(b) Enantiomers:
Non super-imposable mirror images are known as enantiomers. e.g., D and L sugars.
Explanation:
When white light (which is a mixture of different wavelengths) is passed through a Nichol prism, then the emerging light will be of a single wavelength and this is known as plane polarized light. When this plane polarized light is passed through a solution containing carbohydrate, the light is deflected either towards the right or left, which depends upon the configuration of atoms around the chiral centre.
When the solution containing glyceraldehyde with the configuration in the figure given below around the chiral centre is taken, wherein the —OH group on the asymmetric carbon atom is towards right, when written on paper in the straight line projection form, then the light is deflected towards right. Hence this glyceraldehyde is known as dextrorotatory sugar or compound and is designated as d-sugar (+ sugar).
When the solution containing glyceraldehyde with the following configuration around the chiral centre is taken, wherein the —OH group on the asymmetric carbon atom is towards left, when written on paper in the straight line projection form, then the plane polarized light is rotated towards left. Hence this glyceraldehyde is known as levorotatory sugar or compound and is designated as 1-sugar (— sugar).
Glyceraldehyde has only one chiral centre or asymmetric carbon atom, whereas tetroses, pentose’s and hexoses have more than one chiral centre. In such cases the rotation of the plane polarized light is dependent upon many factors, viz., the configuration around each of the chiral centre, the solvent in which it is present, temperature, etc.
Under such circumstances there is no relation between the configurations of the sugar to the rotation of the plane polarized light. Hence other compounds (sugars, amino acids, etc.) are grouped into two categories namely D series compounds and L series compounds.
D series compounds are those compounds that contain the reference group on the right side of the last chiral centre from the functional group. If glucose is taken, the functional group is the aldehydic group (—CHO) and the chiral centre furthermost from it is the 5th carbon atom and the reference group -OH is present on the right side of the straight chain.
Hence it is known as D-glucose. This glucose may or may not be dextrorotatory. It may also be levorotatory. If this glucose is dextrorotatory then it is designated as D-(+)-glucose and if this glucose is levorotatory then it is designated as D-(-)-glucose.
L series compounds are those compounds that contain the reference group on the left side of the last chiral centre from the functional group. If the -OH group is present at the left side on 5th carbon of the straight chain form of glucose then it is known as L-glucose. This glucose may or may not be levorotatory. It may also be dextrorotatory. If this glucose is dextrorotatory, then it is designated as L-(+)-glucose and if this glucose is levorotatory then it is designated as L-(-)-glucose.
Racemic mixture:
A solution containing equal number of d (+) & l (-) forms of a sugar is known as a racemic mixture.
(c) Anomers:
Sugars differing at the anomeric carbon atom are known as anomers.
Explanation:
When an aldehydic group (or carbonyl carbon) reacts with an alcoholic group, then it results in the formation of a hemiacetal. Carbohydrates contain both aldehydic (carbonyl) and alcoholic groups within the molecule. Hence it is possible that the aldehydic group present at the 1st carbon atom of the sugars can react with any of the alcoholic groups present on the other carbon atoms, thus resulting in the creation of an additional chiral centre at the 1st carbon atom and this chiral centre is now known as the anomeric carbon atom. Sugars differing at this anomeric carbon atom are known as anomers.
Two anomers for each of the sugars are possible. If the -OH group on the anomeric carbon atom is towards the right then it is known as alpha (α) anomer. If the —OH group on the anomeric carbon atom is towards the left, then it is known as beta (β) anomer or β-sugar.
3. Ring structures of carbohydrates:
Aldehydic group on 1st carbon atom of sugars can react with the alcoholic group on 4th carbon atom in pentose’s and 4th or 5th carbon atoms in hexoses, forming an hemiacetal (as explained under anomers). This results in the formation of a cyclic ring structure. If the 1st and the 4th carbon atoms are involved in the hemiacetal formation, then the resultant ring structure is a five membered ring that resembles another compound known as furan. Hence the name of the resultant carbohydrate ring structure is furanose ring.
If the 1st and the 5th carbon atoms of the same sugar are involved in the hemiacetal formation then the resultant ring structure is a five membered ring that resembles another compound known as pyran. Hence the name of the resultant carbohydrate ring structure is pyranose ring.
Among the carbohydrates, trioses and tetroses do not involve in the ring formation owing to their short length. Pentose’s always forms the furanose ring structure, whereas hexoses can form both furanose and pyranose ring structures. The following are the ring structures of a few monosaccharide’s.
4. Mutarotation:
Change in the specific rotation of an optically active compound without any change in its other properties is knows as mutarotation.
Explanation:
Glucose crystallized from cold water is α-D-glucose and it shows a specific rotation of {α}20D = +112.2°. If it is dissolved in water, the specific rotation gradually changes with time and reaches a stable value of 52.7°.
This change in specific rotation is because α-D-glucose isomerizes to β-D-glucose via a straight chain intermediate and finally an equilibrium mixture of about 1/3rd of α-D-glucose, 2/3rds of β-D- glucose and a little of straight chain form is formed. This change in specific rotation is known as mutarotation.
Similarly, β-D-glucose, which can be obtained on crystallization from pyridine shows a specific rotation of {α}20D = +19°. When this is dissolved in water its rotation gradually changes and finals to 52.1°. This is again due to mutarotation and formation of α, β and straight chain forms of glucose in an equilibrium of 1/3: 2/3: 0/1 (n).
Chemical Reactions of Carbohydrates:
1. Reducing action of sugars:
In alkaline medium, the aldehydic or ketonic group of sugars can reduce a number of substances (metals) like copper, silver, mercury and bismuth. Copper salts are reduced to cuprous hydroxide or oxide in solution. The sugars are identified in the urine and blood based upon this principle. Benedict’s reagent is commonly used for the detection of sugars in urine.
(a) Reducing sugars:
Sugars having a free aldehydic or ketonic group are known as reducing sugars, e.g., glucose, fructose, galactose and all other monosaccharide’s. Among disaccharides maltose and lactose are reducing sugars.
Benedict’s test:
This is a semi quantitative test most commonly used for the detection of the percentage of sugar in urine. Benedict’s test is carried out in a mild alkaline media. Hence weak reducing agents like uric acid and creatinine in urine cannot reduce Benedict’s reagent. Therefore, this test is very specific for glucose or other reducing sugars in urine.
Principle:
Cupric ions (hydroxide) in the Benedict’s reagent are kept in solution as alkaline citrate complex. When Benedict’s reagent is heated with the reducing sugar, the cupric ions are reduced to cuprous ions (oxide), which are less soluble in water, and hence they precipitate out of the alkaline solution as cuprous oxide.
Procedure:
5 ml of Benedict’s reagent is taken in a clean dry test tube and it is heated to boil. Upon confirmation that there is no formation of precipitate, 8 drops of urine is added to it and is heated for 2 more minutes. Formation of a coloured precipitate, after addition of urine is a positive indication. The colour of the precipitate depends upon the percentage of reducing sugar.
(b) Non-reducing sugars:
Sugars that do not have a free aldehydic or ketonic group are called as non-reducing sugars, e.g., sucrose and trehalose. Note: Though polysaccharides have at least one free aldehydic or ketonic group, but still they are non- reducing sugars owing to their larger molecular size and complexity of the structure. Hence the aldehydic or ketonic group is not available for the reducing action.
2. Formation of osazones:
Phenyl hydrazine reacts with reducing sugars to form osazones. It involves carbonyl carbon and the adjacent carbon. Osazone is a crystalline compound and is used as an identification test for sugars.
Fructose and glucose forms a broom stick shaped crystal in 3 and 5 minutes respectively. Maltose forms star shaped crystals in 20 minutes whereas lactose forms puff shaped crystals in 30 minutes time.
3. Oxidation of sugars:
(a) Mild oxidizing agent like bromine oxidizes the aldehydic group of carbohydrates converting it to an acid group.
(b) Strong oxidizing agent like nitric acid oxidizes the primary alcohol of the carbohydrates forming saccharic acids.
Galactose forms mucic acid, which in insoluble in water. This forms an identification test for galactose known as mucic acid test.
(c) Enzymes:
Inside the cell, the enzymes oxidize both the aldehydic and primary alcoholic groups of the carbohydrates forming uronic acids.
D-glucuronic acid is a component of structural materials like chondroitin sulphate, mucoitin sulphate and glycoproteins (proteoglycons). It plays an important role in detoxification of bile pigments. L-glucose forms ioduronic acid.
4. Dehydration with strong acids:
Concentrated H2SO4 removes the adjacent —OH groups as water (H2O) forming furfural form pentose’s and hydroxymethyl furfural from hexoses.
Furfural condenses with α-naphthol in presence of alcohol forming a purple violet coloured complex. This is the principle of Molisch’s test which is a common identification test for all carbohydrates.
5. Derived sugars:
Substances formed from sugars on oxidation, reduction or addition/replacement of any group are called derived sugars.
(a) Amino sugars:
The hydroxyl group at the second carbon of a sugar is replaced by an amino group to form an amino sugar, e.g., glucosamine, galactosamine.
(b) Deoxy sugars:
These sugars are formed due to removal of one of the oxygen from the alcoholic group, e.g., 2-Deoxy-ribose, here the ‘O’ of the 2nd alcoholic group is removed. It is present in DNA. L-fucose is 6-Deoxy L-galactose, L-rhamnose is 6-Deoxy L-mannose.
(c) Oxidation products of carbohydrates:
Uronic acids and saccharic acids are also derived sugars.
6. Formation of glycosides with alcohol:
When two alcoholic groups react with each other, a glycoside is formed. Carbohydrates contain many alcoholic groups. Hence two carbohydrates can react with alcoholic groups of one another sugars, forming glycosides. Union of two carbohydrates is known as a disaccharide, three is trisaccharide and many is a polysaccharide.
Medically important glycosides:
There are some glycosides, other than carbohydrates discussed in this article, that are medically important, some of them are:
(i) Digitonin:
This is a cardiac glycoside.
(ii) Saponin:
A plant glycoside used as an immuno-stimulating agent.
(iii) Phlorhizin:
Also a plant glycoside used in kidney functions.
Carbohydrate Type # 2. Disaccharides:
Sugars containing two monosaccharide units linked by glycosidic bond are known as disaccharides.
The three most common disaccharides are discussed below:
1. Maltose:
It contains two a-D-glucose units linked by α-1 → 4 glycosidic linkage. Chemically it is named as α-D-glucopyranosyl-(α-1 → 4)-α-D-glucopyranose. It is also known as malt sugar. It is the product of starch hydrolysis. It is a reducing sugar and forms star shaped osazone crystals.
2. Lactose:
Made up of β-D-galactopyrasone and α-D-glucopyranose linked through β-1 → 4 glycosidic linkage. Chemically it is called as β-D-galactopyranosyl-(β-1 → 4)-α-D-glucopyranose. It is present in milk and hence called milk sugar. It is a reducing sugar and forms puff shaped osazone crystals.
3. Sucrose:
Contains α-D-glycopyranse and β-D-fructofuranose linked through α-1 → 2 glycosidic linkage. Its chemical name is α-D-glucopyranosyl-(α-1 → 2)-β-D-fructofuranose. It is the common table sugar obtained from sugar cane hence the name cane sugar. As it is a non-reducing sugar it does not form osazones. It is also known as invert sugar.
Invert sugar:
Sucrose is known as invert sugar because sucrose is dextrorotatory with a specific rotation of +62.5°. On hydrolysis by an enzyme – sucrase or invertase, it gives a mixture of glucose and fructose. This mixture exhibits a net specific rotation of —19°, i.e., levorotatory. The phenomenon by which dextrorotatory sugar is converted to a levorotatory sugar is known as invert sugar.
Chocolate companies use invertase enzyme in the preparation of toffees in order to increase the taste and commercial value. In the preparation of these toffees solid sucrose caramel is made and coated with the enzyme-sucrase or invertase over which cocoa is engorged. The enzyme hydrolyses solid sucrose into liquid glucose and fructose which is seventy times sweeter, thereby attracting the consumer.
Carbohydrate Type # 3. Polysaccharides:
Carbohydrates made up of 10 or more monosaccharide units are called as polysaccharides. They are also known as glycans. They are further classified as homopolysaccharides and heteropolysaccharides.
Homopolysaccharides:
Those polysaccharides which contain only one kind of monosaccharide unit are called homopolysaccharides. e.g., starch, glycogen, cellulose, dextran, inulin, agar, chitin, etc.
1. Glycogen:
It is known as animal starch. It is made up of α-D-glucose units linked by α-1 → 4 linkages in the linear and α-1 → 6 linkages at the branching points. It is highly branched. Glycogen is the storage form of energy (glucose) in each and every cell of the human body. Liver and muscle contain the highest amount of glycogen. At least 5% of glycogen is present in each cell even under severe fasting/starvation condition. It gives a red colour with iodine.
2. Starch:
It is made up of α-D-glucose units, hence known as glucosan. It is composed of amylose and amylopectin. Amylose is coiled and un-branched. The α-D-glucose units are linked by glycosidic linkages. Amylopectin is uncoiled and is highly branched. It has α-1 → 4 linkages in the linear chain and α-1 → 6 at the branching points. It is the chief carbohydrates present in plants and forms the main source of dietary energy sources to humans. It gives a blue colour with iodine.
3. Cellulose:
Made up of β-D-glucose units linked by β-1 → 4 glycosidic linkages. It is un-branched. It is the most abundant carbohydrate in nature. It forms the woods of the plant. Cellulase enzyme is absent in human being and hence it becomes non-utilizable. However, it adds to the bulk of the food and helps in the gastric motility.
4. Dextran:
It is produced by yeasts and bacteria. It is made up of α-D-glucose linked by α-1 → 6 glycosidic linkages. The branching points are at 1-2, 1-3 and 1-4. It absorbs water to form gels. It is used as plasma substitute.
5. Inulin:
It is a fructosan. It cannot be metabolized by the body, hence used to assay glomerular filtration rate (G.F.R.) in the study of kidney function.
6. Agar:
It is a sulphated galactose. It dissolves in hot water. It gels on cooling, thereby forming a solidified medium in tissue culture studies.
7. Chitin:
N-acetylglucosamine (chitosamine) linked by β-1 → 4 linkages. Present in the exoskeleton of invertebrates like cockroach and crab.
Heteropolysaccharides:
Polysaccharides made up of two or more kinds of monosaccharide units, e.g., pectin’s and mucopolysaccharides.
Most of them are branched and exist in conjugation with proteins and hence called proteoglycans. The carbohydrate part is called glycosaminoglycan. They have a repeating disaccharide unit which is acetylated or sulphated. There are two major types of heteropolysaccharides.
(a) Pectins:
They are composed of galacturonic acid, galactose and arabinose.
(b) Mucopolysaccharides:
They are sticky polysaccharides (mucin like).
The various types of mucopolysaccharides and their composition is given below: